Is Vital Flex Core Fda Approved

Imagine a world where back pain no longer dictates your daily routine, where you can bend, lift, and twist without wincing. For many, that world seems attainable with the promise of products like the Vital Flex Core, a device marketed towards alleviating back discomfort. But behind the enticing claims and testimonials, a crucial question lingers: Is it legit, and has it earned the stamp of approval from the FDA?
The surging popularity of back pain solutions has led to a market flooded with various gadgets and therapies. Understanding whether the Vital Flex Core has received FDA approval is critical for consumers to make informed decisions about their health and well-being. This article delves into the details surrounding this device, its claims, and its FDA status, providing a comprehensive overview to help you navigate the complexities of back pain relief solutions.
Unpacking the Vital Flex Core
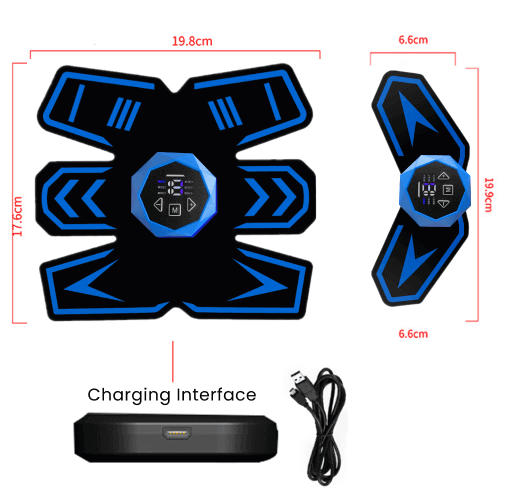
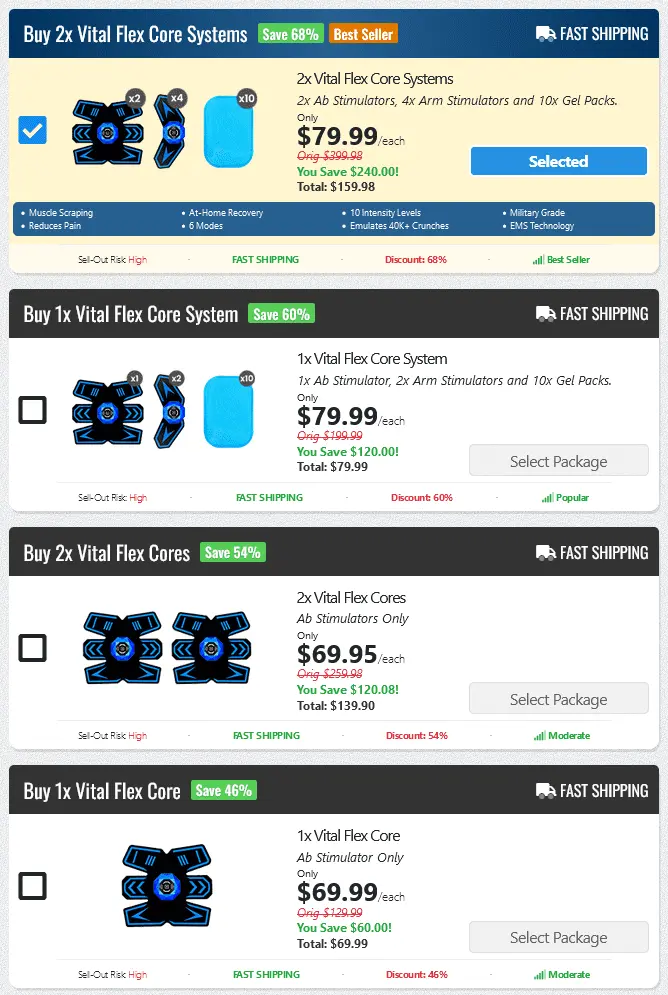
The Vital Flex Core is often advertised as a revolutionary device designed to relieve back pain and improve posture. It's presented as a non-invasive solution that can be used at home, offering a convenient alternative to traditional treatments. Many testimonials highlight its potential to reduce discomfort, enhance flexibility, and promote overall spinal health.
The device typically involves a combination of support and potentially heat or massage features. The intention is to target key muscle groups in the back and core, aiming to alleviate pain and improve stability. Advertisements often emphasize the ease of use and the potential for long-term benefits.
The FDA's Role: Guardian of Public Health
The Food and Drug Administration (FDA) plays a vital role in ensuring the safety and effectiveness of medical devices sold in the United States. The FDA regulates medical devices based on their risk level, classifying them into three categories: Class I, Class II, and Class III.
Class I devices pose the lowest risk and include items like bandages and manual stethoscopes. Class II devices present moderate risk and often require premarket notification (510(k) clearance). Class III devices are the highest risk and usually require premarket approval (PMA) before they can be sold to the public.
Premarket approval involves a rigorous review process, including clinical trials and extensive data analysis. This process is designed to ensure that the device is both safe and effective for its intended use. FDA approval signifies that the device has met specific standards and can be legally marketed.
Is Vital Flex Core FDA Approved? The Search for Clarity
Determining whether the Vital Flex Core has FDA approval requires careful investigation. Searching the FDA's database is the first step. The FDA's website allows users to search for registered medical devices.
Based on current information available in the FDA's databases and general understanding of device classification, it's important to note that many back pain relief devices fall under Class I or Class II. Class I devices are typically exempt from premarket notification, while Class II devices require 510(k) clearance.
The absence of explicit FDA approval for a product like the Vital Flex Core doesn't automatically mean it's unsafe or ineffective. It may indicate that the device falls under a category that doesn't require premarket approval, or that it has obtained 510(k) clearance, which demonstrates substantial equivalence to a legally marketed device. However, it's also crucial to critically evaluate the available evidence supporting the product's claims.
Navigating the Claims and Promises
When evaluating any health product, especially those claiming to alleviate pain, it's essential to approach the marketing claims with a critical eye. Testimonials can be compelling, but they are often anecdotal and may not reflect the experience of all users.
Look for scientific evidence that supports the claims made by the manufacturer. Check if the device has been studied in clinical trials, and if the results have been published in peer-reviewed journals. Consider the source of the information and whether it is biased or objective.
Consult with a healthcare professional before using any new device or treatment for back pain. A doctor or physical therapist can assess your condition and recommend the most appropriate course of action. They can also help you evaluate the claims made by the Vital Flex Core and determine if it is likely to be beneficial for you.
Alternative Approaches to Back Pain Relief
While devices like the Vital Flex Core might offer some relief, it's important to explore a range of evidence-based approaches to managing back pain. Physical therapy is a cornerstone of back pain treatment.
Exercises designed to strengthen core muscles and improve flexibility can significantly reduce pain and prevent future problems. Over-the-counter pain relievers, such as ibuprofen and acetaminophen, can also provide temporary relief. In some cases, prescription medications may be necessary.
Lifestyle modifications, such as maintaining a healthy weight and practicing good posture, can also play a crucial role in managing back pain. Techniques like yoga and meditation can help reduce stress and muscle tension, contributing to overall well-being.
The Consumer's Role: Due Diligence and Informed Choices
Ultimately, the responsibility of making informed decisions about health products rests with the consumer. Before purchasing any device, do your research. Read reviews from multiple sources and consider both positive and negative feedback.
Check the manufacturer's website for detailed information about the product, including its features, intended use, and any potential risks. Look for certifications or endorsements from reputable organizations. Consult with healthcare professionals to gain personalized recommendations.
By taking a proactive approach and critically evaluating the available information, you can make choices that are aligned with your health goals and needs.
A Final Reflection
The allure of quick and easy solutions to persistent problems like back pain is understandable. Products like the Vital Flex Core tap into that desire, offering the promise of relief and improved quality of life. However, the path to lasting wellness requires careful consideration and a balanced approach.
While the FDA's role in regulating medical devices is vital, it's equally important for individuals to educate themselves and engage in informed decision-making. By combining scientific knowledge with professional guidance, we can navigate the complex landscape of health and wellness and achieve meaningful improvements in our lives.
The journey to a pain-free back may not always be straightforward, but with the right tools and a proactive mindset, it's a journey worth undertaking. Remember to prioritize your health, seek expert advice, and approach every new solution with a healthy dose of skepticism and a commitment to evidence-based practices.