Leading Gxp Erp Systems For Pharmaceuticals
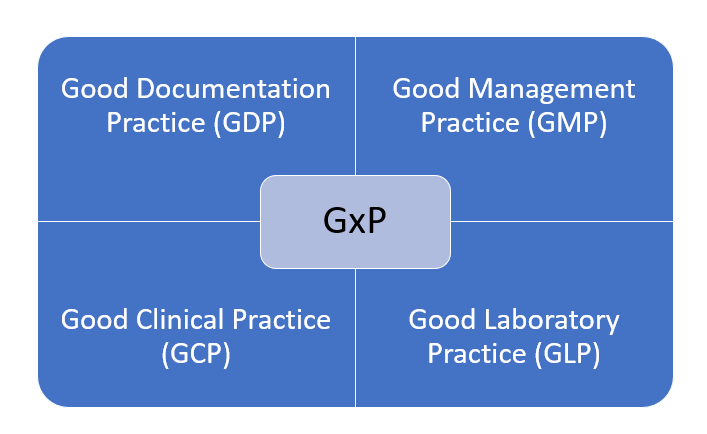
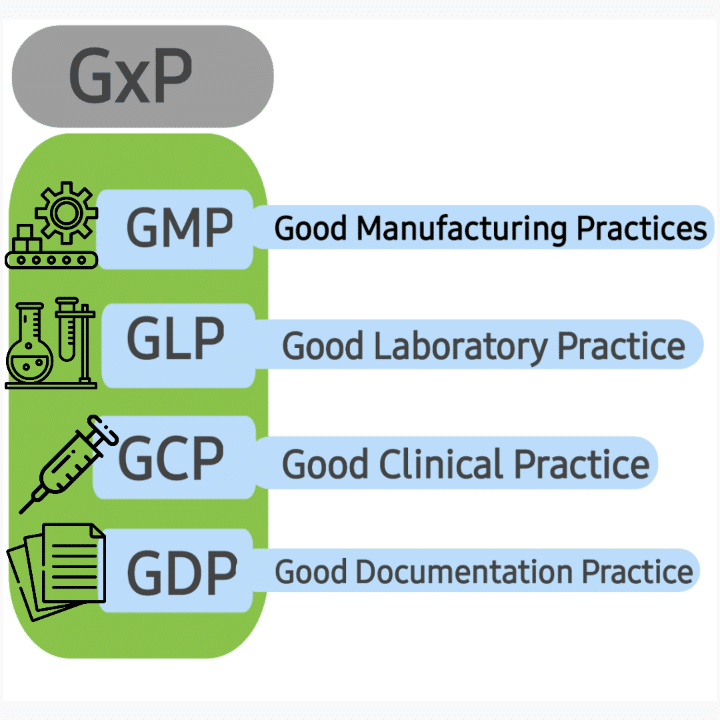
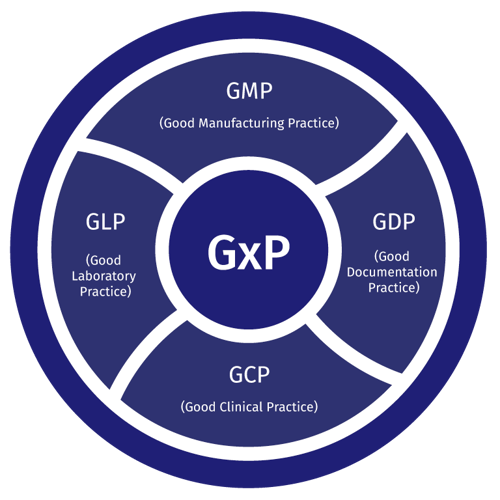
The pharmaceutical industry, known for its rigorous regulatory landscape, heavily relies on GxP (Good Practice) guidelines to ensure the safety, efficacy, and quality of its products. Within this complex environment, Enterprise Resource Planning (ERP) systems tailored for GxP compliance have become indispensable tools.
These specialized ERPs help pharmaceutical companies manage everything from research and development to manufacturing and distribution, all while adhering to stringent regulatory requirements. The adoption of these systems is not merely a matter of efficiency; it's a cornerstone of maintaining patient safety and avoiding costly compliance failures.
Understanding GxP ERP Systems
GxP ERP systems are not generic software solutions. They are specifically designed to address the unique needs of the pharmaceutical industry. They are validated systems that ensure data integrity, audit trails, and security features required by regulatory bodies such as the Food and Drug Administration (FDA) in the US and the European Medicines Agency (EMA) in Europe.
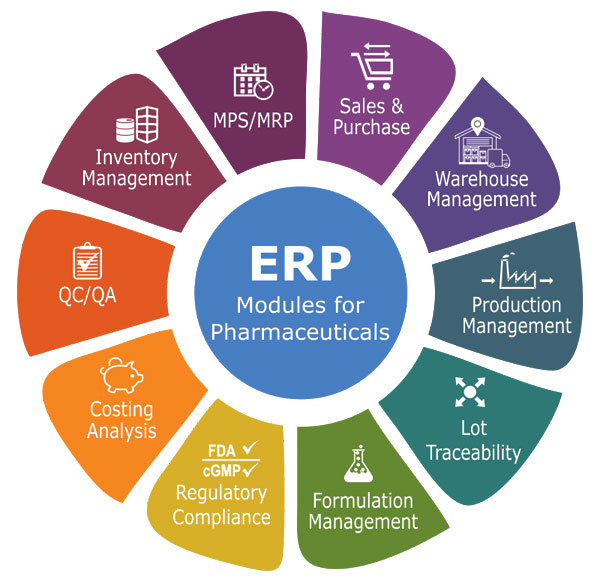
Key features often include robust document management, version control, electronic signatures, and automated workflows designed to minimize human error and ensure traceability. These features are crucial for maintaining compliance with regulations like 21 CFR Part 11, which governs electronic records and signatures.
Leading Vendors and Their Solutions
Several ERP vendors have established themselves as leaders in the GxP-compliant pharmaceutical ERP space. SAP S/4HANA is a prominent player, offering a comprehensive suite of modules that cover all aspects of the pharmaceutical value chain. SAP emphasizes its ability to integrate data across the organization, providing a unified view of operations.
Oracle JD Edwards EnterpriseOne is another widely used solution. Oracle provides a broad range of capabilities including manufacturing, finance, and supply chain management, all designed with GxP compliance in mind.
Beyond these industry giants, specialized vendors like BatchMaster ERP and Deacom (now part of ECI Software Solutions) offer solutions tailored to specific pharmaceutical processes. They often cater to smaller or mid-sized companies that require a more focused approach.
Implementation Challenges and Best Practices
Implementing a GxP ERP system is a complex undertaking. It requires meticulous planning, thorough validation, and ongoing maintenance. Companies must carefully select a vendor that understands the pharmaceutical industry's specific requirements and has a proven track record of successful implementations.
Data migration is a critical phase, ensuring that all existing data is accurately transferred to the new system. Proper training is essential to ensure that all employees understand how to use the system effectively and in compliance with GxP regulations.
Validation is not a one-time event but an ongoing process. Regular audits and system updates are necessary to maintain compliance. A robust change management process is also crucial to ensure that any modifications to the system are properly documented and validated.
The Impact on Pharmaceutical Companies
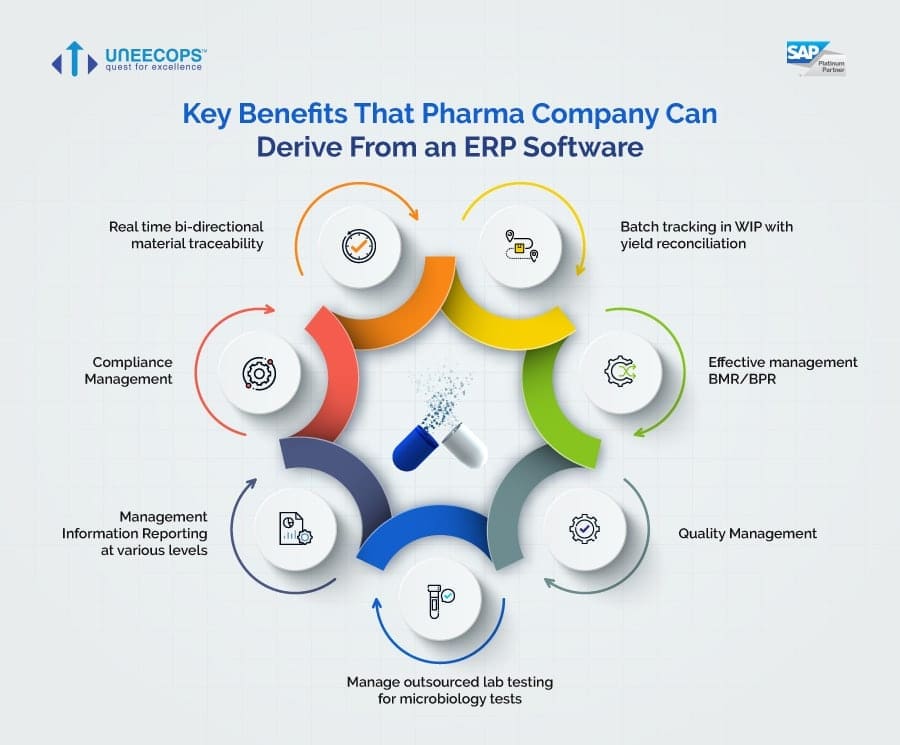
The benefits of implementing a GxP ERP system are significant. Improved data integrity reduces the risk of errors and compliance violations. Enhanced traceability allows for faster and more efficient investigations in the event of a quality issue.
Streamlined processes lead to increased efficiency and reduced costs. Better visibility across the supply chain improves inventory management and reduces the risk of stockouts. Real-time data analytics enable better decision-making and improved overall performance.
Perhaps most importantly, GxP ERP systems help pharmaceutical companies maintain patient safety. By ensuring the quality and integrity of their products, these systems contribute to the overall well-being of patients. This aligns with the industry's core mission of providing safe and effective medicines.
The Future of GxP ERP
The future of GxP ERP is likely to be shaped by several trends. Cloud-based solutions are becoming increasingly popular, offering greater flexibility and scalability. Artificial intelligence (AI) and machine learning (ML) are being integrated into ERP systems to automate tasks and improve decision-making.
The increasing focus on data security is driving the development of more robust security features. Blockchain technology is being explored as a way to enhance supply chain transparency and prevent counterfeiting.
As the pharmaceutical industry continues to evolve, GxP ERP systems will play an increasingly important role in ensuring compliance, improving efficiency, and protecting patient safety. The continuous development and refinement of these systems are essential for supporting the industry's mission of providing safe and effective medicines to patients around the world. For any pharmaceutical company operating in a global marketplace, embracing leading GxP ERP systems is not just a best practice, it is a necessity for sustained success.