Limb Girdle Muscular Dystrophy Competitive Analysis

The race to develop effective treatments for Limb Girdle Muscular Dystrophy (LGMD), a group of inherited diseases causing progressive muscle weakness, is intensifying. Several pharmaceutical companies and research institutions are vying to bring groundbreaking therapies to market, marking a pivotal moment for patients and their families.
This article provides a competitive analysis of the LGMD therapeutic landscape, highlighting key players, therapeutic approaches, and the potential impact of these advancements on the future of LGMD treatment.
Understanding Limb Girdle Muscular Dystrophy
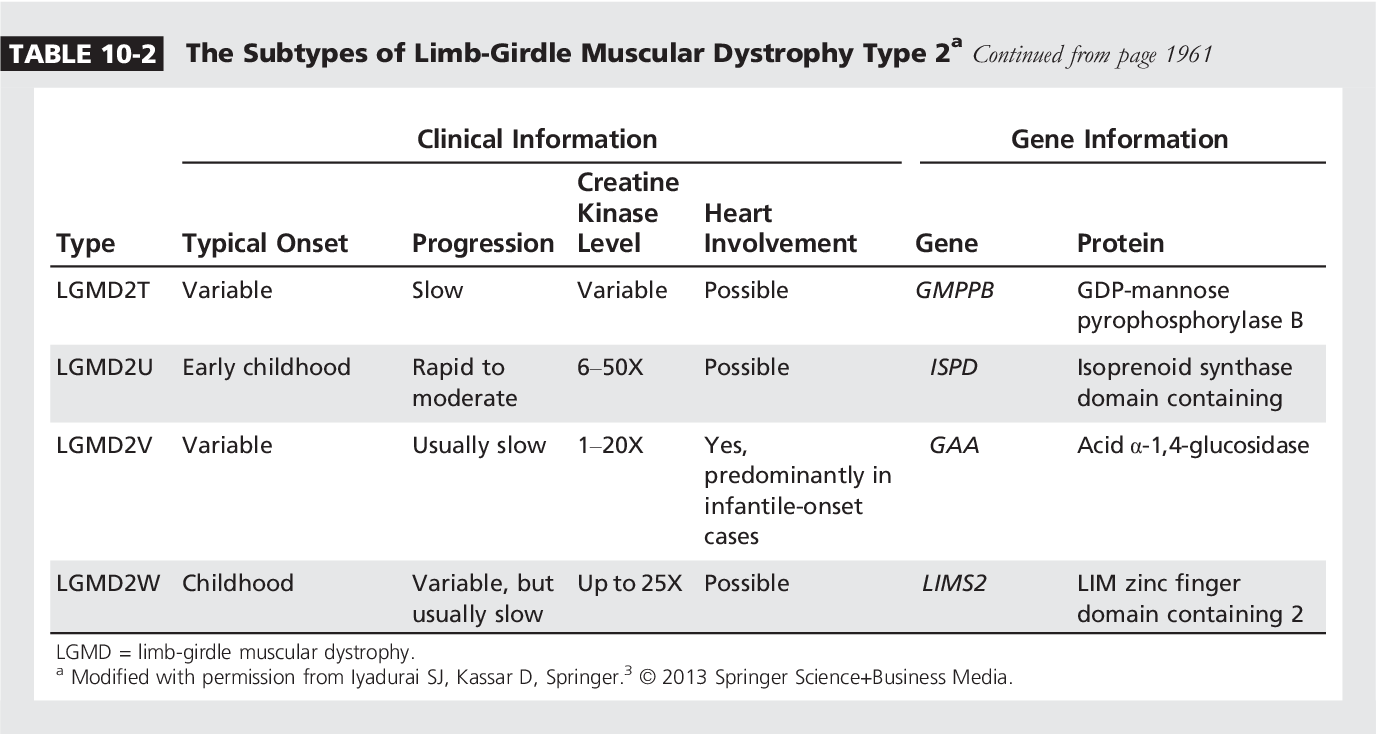
LGMD is not a single disease but a collection of over 30 distinct genetic disorders. These disorders primarily affect the muscles around the hips and shoulders (the limb girdles), leading to difficulty with movement, such as walking, climbing stairs, and raising arms.
The severity and progression of LGMD vary widely depending on the specific gene mutation involved. This heterogeneity presents a significant challenge for developing broadly applicable therapies.
The Competitive Landscape: Key Players and Approaches
Several pharmaceutical companies are actively developing treatments for specific subtypes of LGMD. These efforts primarily focus on gene therapy, exon skipping, and small molecule therapies.
Gene Therapy: A Promising Avenue
Gene therapy aims to correct the underlying genetic defect by delivering a functional copy of the mutated gene into the muscle cells. Sarepta Therapeutics is a leading player in this space, developing gene therapies for several LGMD subtypes.
Their lead candidate targets LGMD2E (also known as beta-sarcoglycanopathy). Initial clinical trial data have shown promising results in improving muscle function and reducing disease progression.
Solid Biosciences is also pursuing gene therapy approaches for specific LGMD subtypes. They are leveraging their expertise in AAV (adeno-associated virus) vectors to deliver therapeutic genes directly to affected muscles.
Exon Skipping: Tailoring Treatments to Specific Mutations
Exon skipping is another approach being explored. This strategy involves using antisense oligonucleotides (ASOs) to "skip" over mutated exons during RNA splicing, resulting in a shortened but functional protein.
While exon skipping has shown some success in Duchenne muscular dystrophy, its application to LGMD is still in early stages. NS Pharma is actively investigating exon skipping therapies for specific LGMD subtypes.
Small Molecule Therapies: Addressing Downstream Effects
Some companies are focusing on small molecule therapies that address the downstream effects of the genetic mutations, rather than directly correcting the gene itself. These therapies aim to improve muscle health, reduce inflammation, or promote muscle regeneration.
Regenxbio, while primarily focused on gene therapy for other neuromuscular disorders, has explored potential small molecule approaches relevant to LGMD. Their research centers on identifying compounds that can enhance muscle function and slow disease progression.
Clinical Trial Landscape and Regulatory Hurdles
The clinical trial landscape for LGMD is rapidly evolving. Several ongoing trials are evaluating the safety and efficacy of different therapeutic approaches.
The U.S. Food and Drug Administration (FDA) and other regulatory agencies are closely monitoring these trials. They're working to establish clear regulatory pathways for LGMD therapies.
The accelerated approval pathway, which allows for faster approval of drugs that address unmet medical needs based on surrogate endpoints, may be relevant for some LGMD therapies.
Challenges and Opportunities
Developing effective treatments for LGMD presents several challenges. These include the genetic heterogeneity of the disease, the limited availability of biomarkers to track disease progression, and the difficulty in conducting clinical trials with small patient populations.
Despite these challenges, there are significant opportunities for advancement. The increasing understanding of the genetic basis of LGMD, coupled with advancements in gene therapy and other technologies, is paving the way for the development of targeted and effective therapies.
Collaboration between researchers, pharmaceutical companies, and patient advocacy groups is crucial to accelerate the development of new treatments. These collaborations would address the unmet needs of individuals living with LGMD.
The Patient Perspective
For individuals and families affected by LGMD, the prospect of new treatments offers hope for a better future. The availability of effective therapies could significantly improve their quality of life, increase their independence, and extend their lifespan.
Patient advocacy groups such as the Cure LGMD2i Foundation and the Muscular Dystrophy Association (MDA) play a vital role in raising awareness of LGMD, supporting research efforts, and advocating for access to care. They also support patients and connect them with clinical trials.
Looking Ahead
The LGMD therapeutic landscape is dynamic and competitive, with several companies actively pursuing innovative treatment strategies. While challenges remain, the progress made in recent years offers hope for a future where effective therapies are available to all individuals affected by this debilitating disease.
The coming years are likely to witness significant advancements in LGMD treatment. This will be thanks to ongoing research, clinical trials, and regulatory approvals.
Continued investment and collaboration are essential to ensure that these advancements translate into meaningful improvements for patients and their families. The collective effort is paving the way for a future where LGMD is no longer a life-limiting condition.