Over The Counter Alternative For Viagra

The pursuit of readily accessible solutions for erectile dysfunction (ED) has taken a significant turn with the emergence of over-the-counter (OTC) alternatives to prescription medications like Viagra. These alternatives, often marketed as dietary supplements, promise similar benefits without the need for a doctor's visit, sparking both interest and concern within the medical community and among consumers.
This article examines the growing market for OTC ED alternatives, focusing on their ingredients, purported benefits, potential risks, and regulatory landscape. It aims to provide a balanced perspective on this evolving area of men's health, drawing on expert opinions and available scientific evidence.
The Rise of OTC ED Alternatives
The market for OTC ED alternatives has expanded rapidly in recent years, driven by a desire for convenience and discretion. Many men are hesitant to discuss ED with their doctors, making OTC options appealing despite the lack of rigorous regulatory oversight.
These products are typically marketed online and in retail stores, often promising improved sexual performance and increased libido. However, the efficacy and safety of these supplements are often questionable.
Key Ingredients and Claims
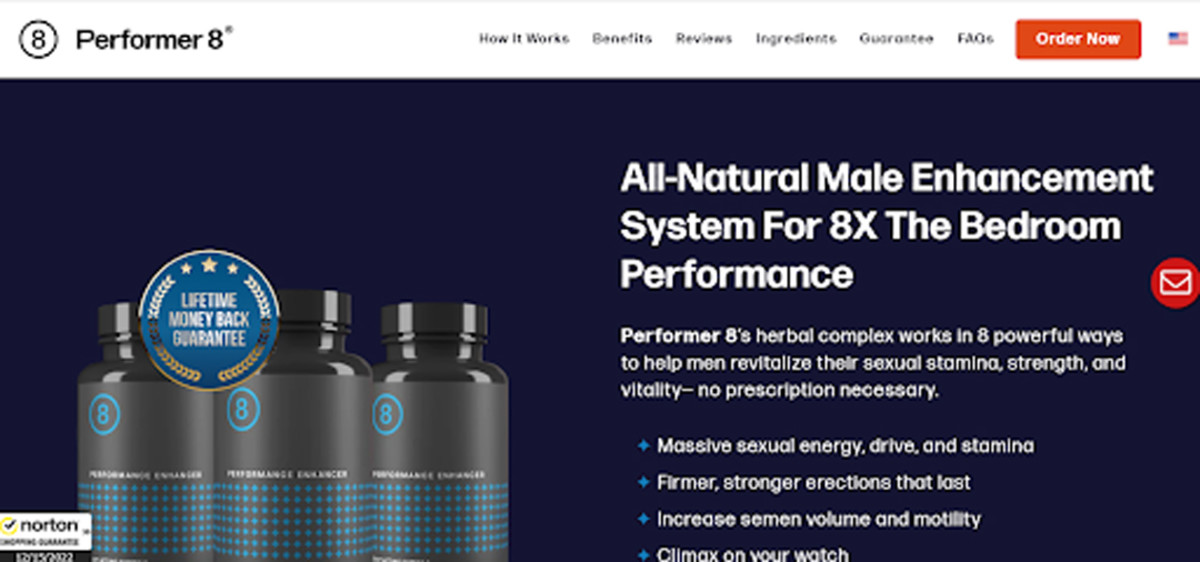
OTC ED alternatives often contain a blend of herbal ingredients, amino acids, and vitamins. Common ingredients include L-arginine, horny goat weed, yohimbe, and various herbal extracts like ginseng and maca.
Manufacturers claim that these ingredients can improve blood flow, boost testosterone levels, and enhance sexual function. However, scientific evidence supporting these claims is often limited or inconclusive.
The National Institutes of Health (NIH) maintains a database of information on dietary supplements, cautioning that "many supplements have not been well studied" and that "some supplements can interact with medications or have other risks."
Potential Risks and Concerns
One of the primary concerns surrounding OTC ED alternatives is the lack of regulatory oversight. Unlike prescription medications, these supplements are not subject to pre-market approval by the Food and Drug Administration (FDA).
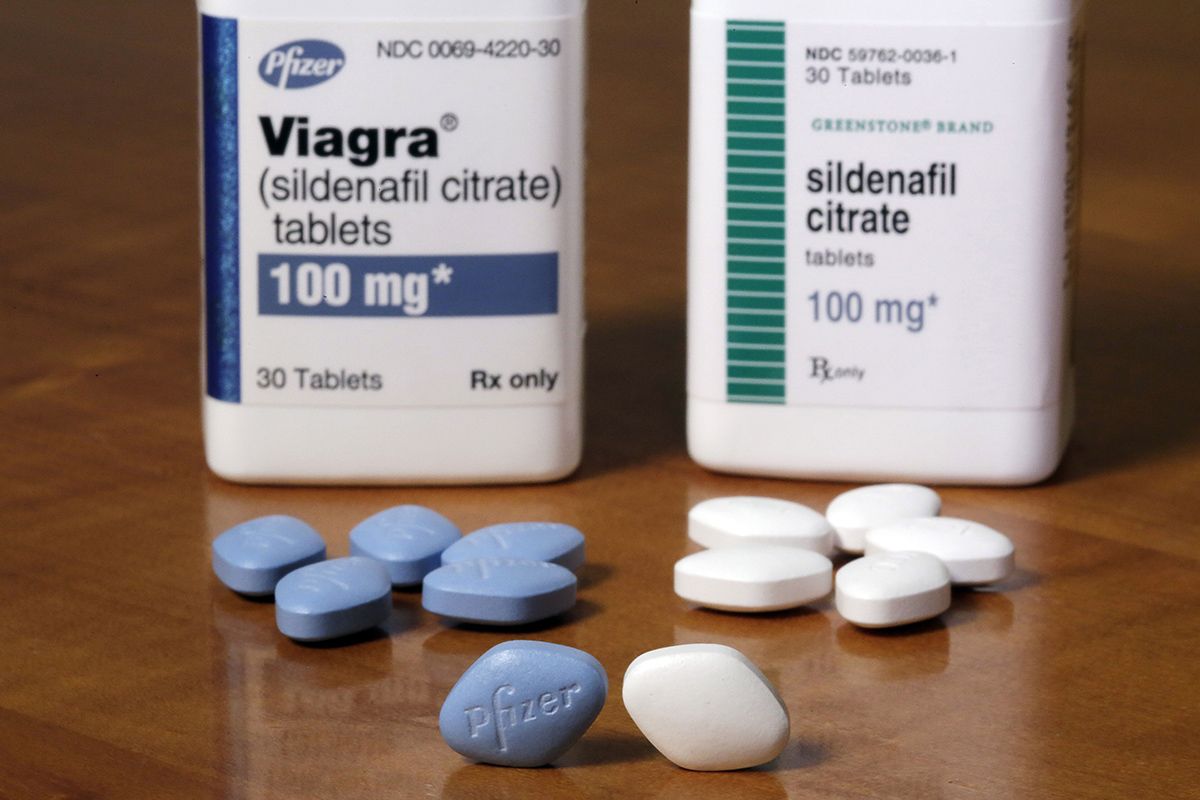
This means that the quality, purity, and potency of these products can vary significantly. The FDA has issued warnings about some OTC ED products containing hidden pharmaceutical ingredients, such as sildenafil, the active ingredient in Viagra, which could pose serious health risks, especially for men with underlying heart conditions or those taking medications containing nitrates.
Additionally, the potential for interactions with other medications and the lack of standardized dosages raise further concerns. Consumers may unknowingly be exposing themselves to unforeseen health risks.
Expert Opinions and Recommendations
Medical professionals generally advise caution when considering OTC ED alternatives. Dr. Emily Carter, a urologist at Mayo Clinic, emphasizes the importance of consulting with a physician before using any ED treatment, including over-the-counter options. "It's crucial to understand the underlying cause of the ED and to rule out any potential medical conditions," she states.
Dr. Carter also warns against the potential for false advertising and unsubstantiated claims. She encourages patients to be skeptical of products that promise quick fixes or miraculous results.
She recommend lifestyle changes like regular exercise, a healthy diet, and stress management as first-line approaches to address ED.
The Regulatory Landscape
The FDA has taken action against some manufacturers of OTC ED products, issuing warning letters and recalls for products found to contain undeclared pharmaceutical ingredients or make false claims. However, the vastness of the market and the ease with which these products can be sold online make enforcement challenging.
The Dietary Supplement Health and Education Act (DSHEA) of 1994 governs the regulation of dietary supplements in the United States. This law places the burden of proof on the FDA to demonstrate that a supplement is unsafe before it can be removed from the market.
This regulatory framework has been criticized for allowing potentially harmful products to remain available to consumers.
Consumer advocacy groups are calling for stricter regulations and increased transparency in the dietary supplement industry. They argue that consumers need better information about the risks and benefits of these products.
Conclusion
The emergence of OTC ED alternatives presents both opportunities and challenges. While these products may offer a convenient and discreet solution for some men, the lack of rigorous regulatory oversight and the potential for undisclosed ingredients raise significant concerns.
Consumers are urged to exercise caution, conduct thorough research, and consult with a healthcare professional before using any OTC ED treatment. A proper diagnosis and personalized treatment plan are essential for addressing ED effectively and safely.
Ultimately, informed decision-making is crucial when navigating the complex world of men's health and sexual wellness.