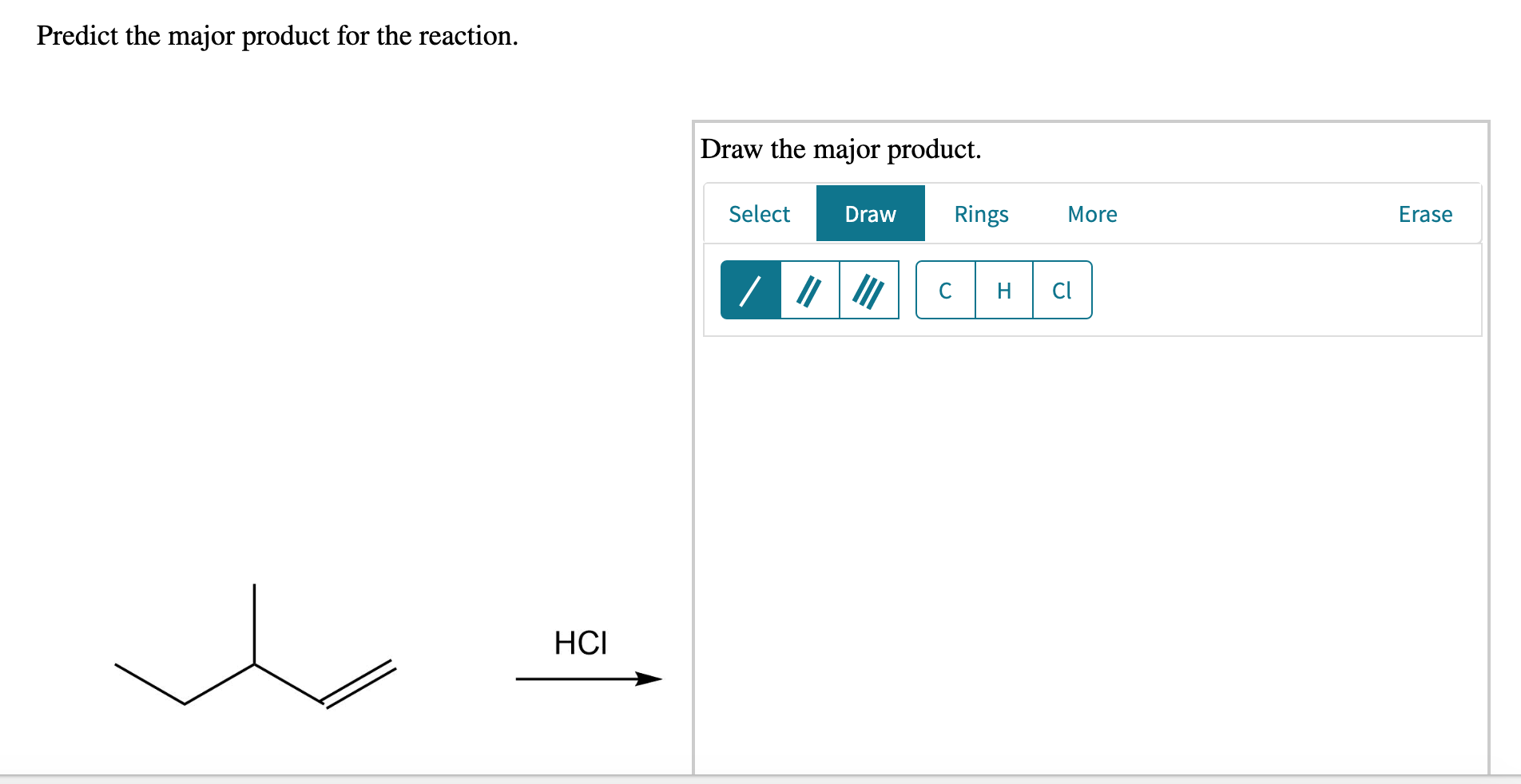
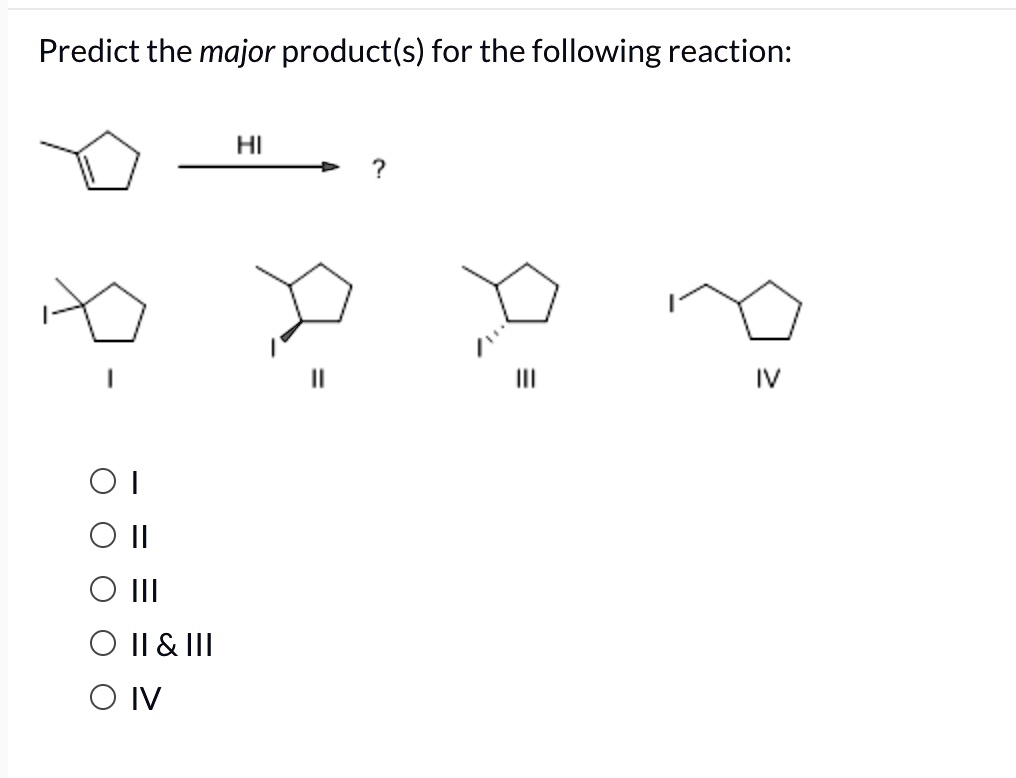
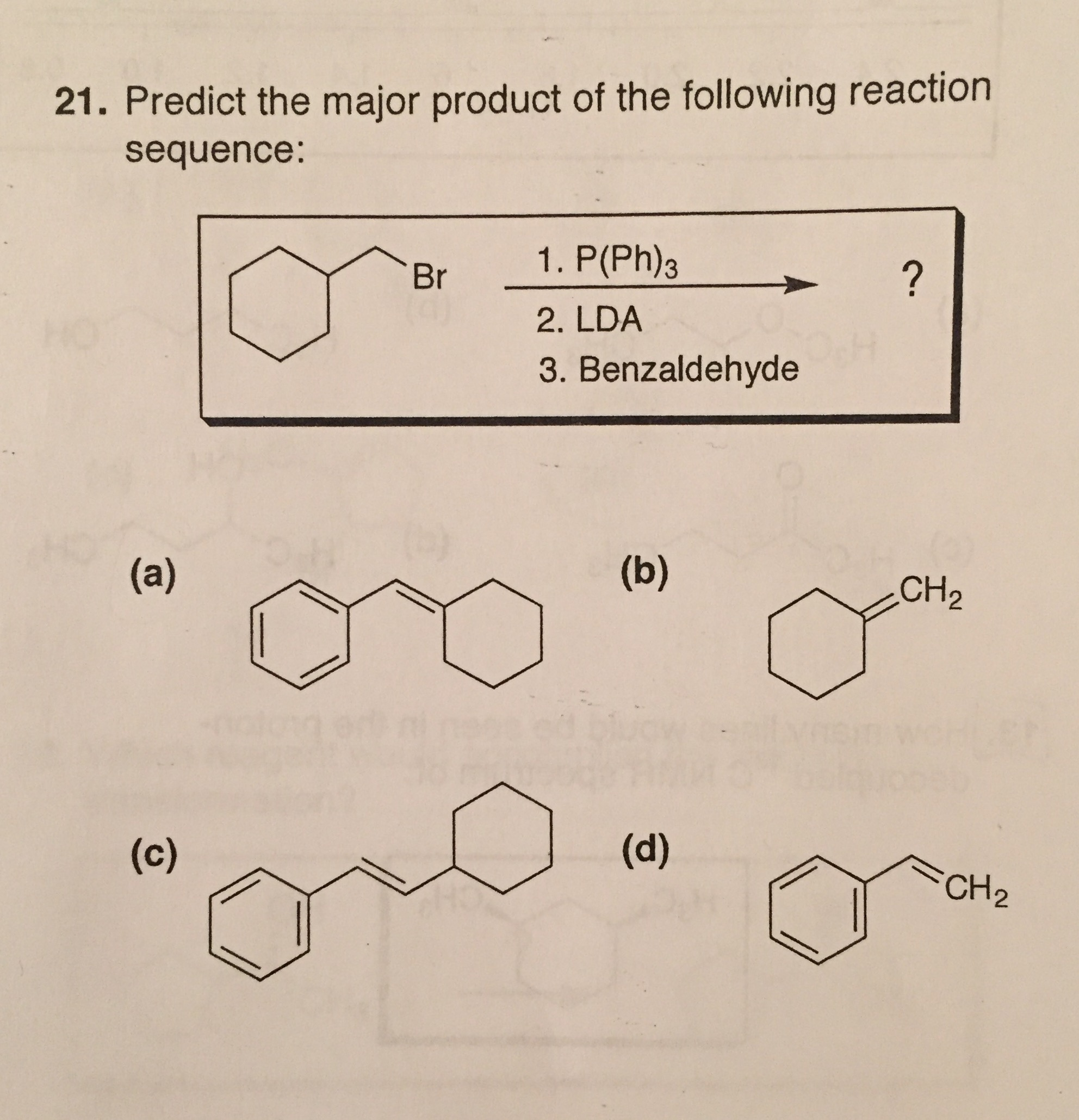
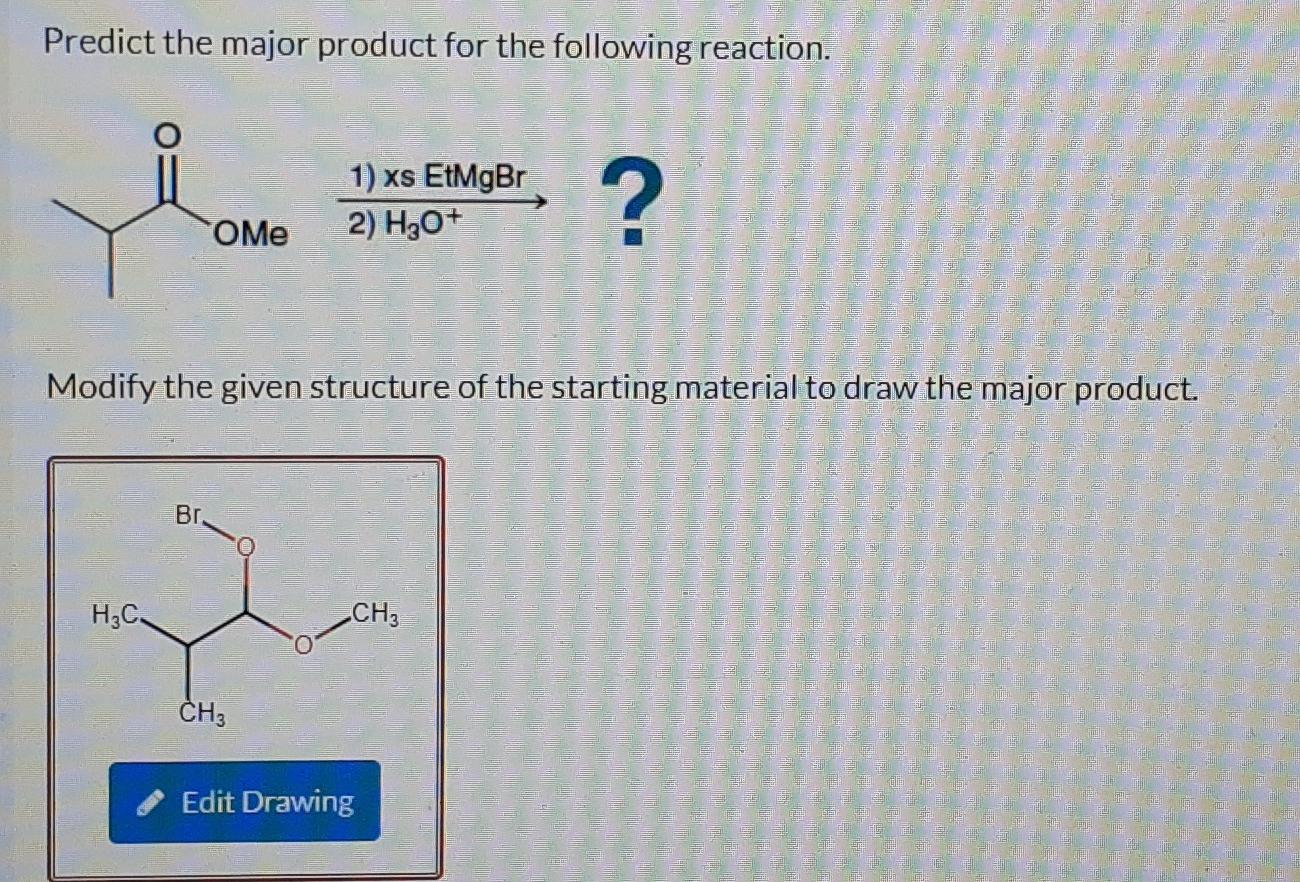
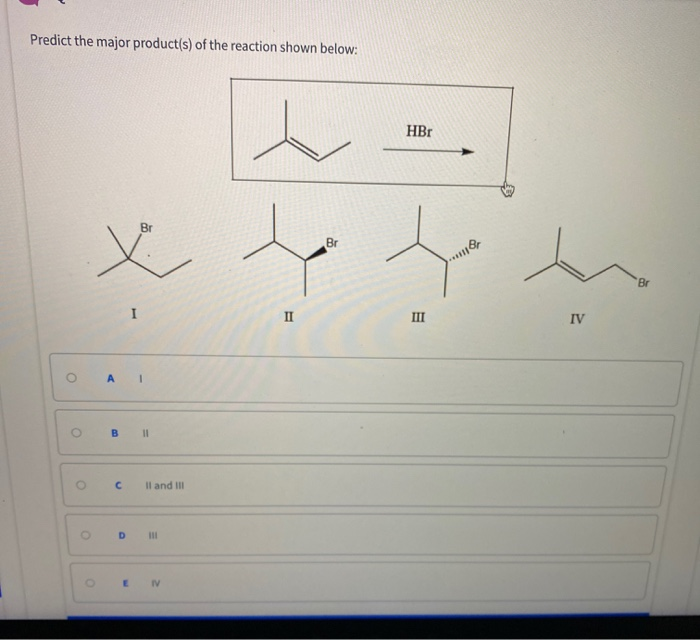
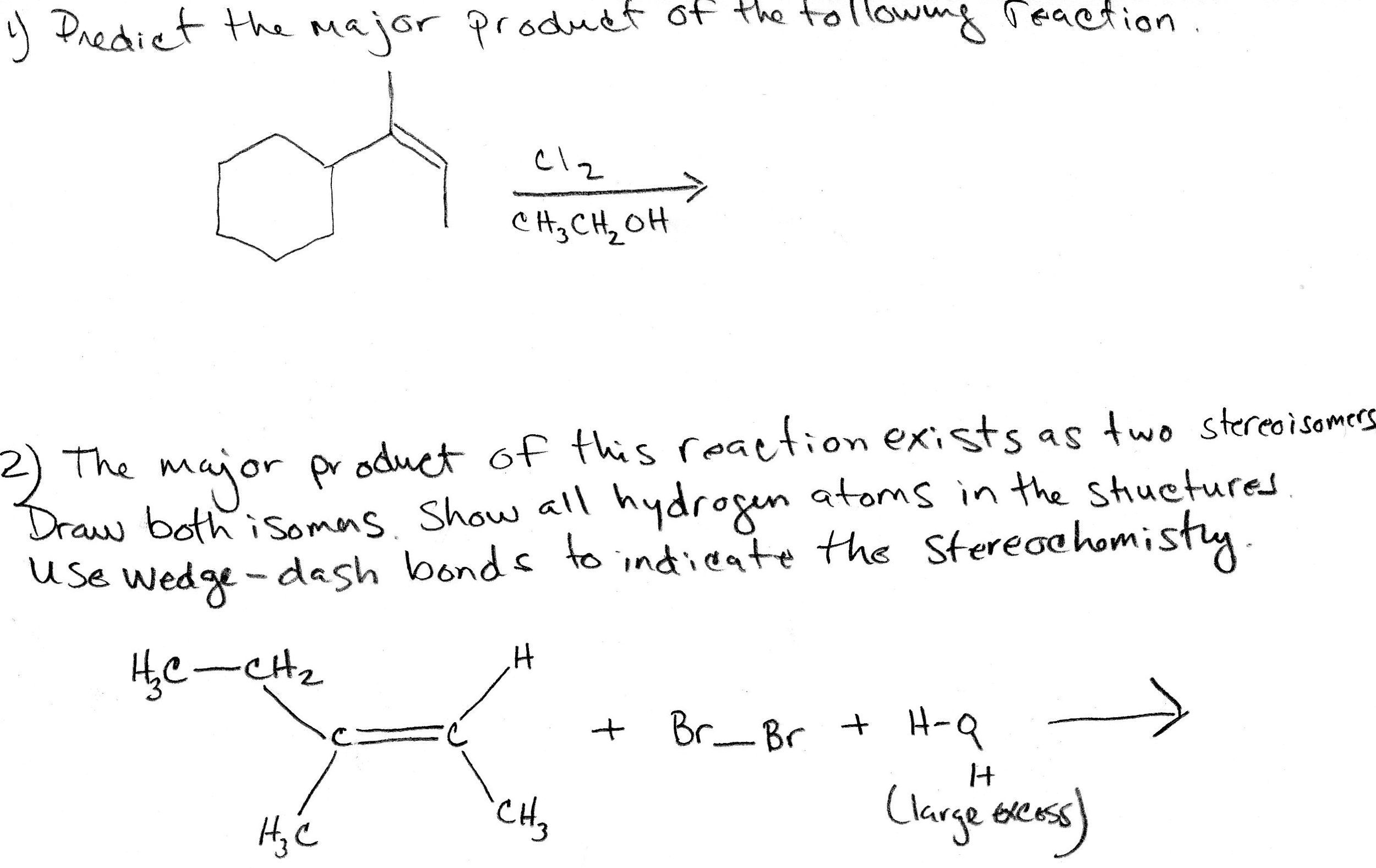
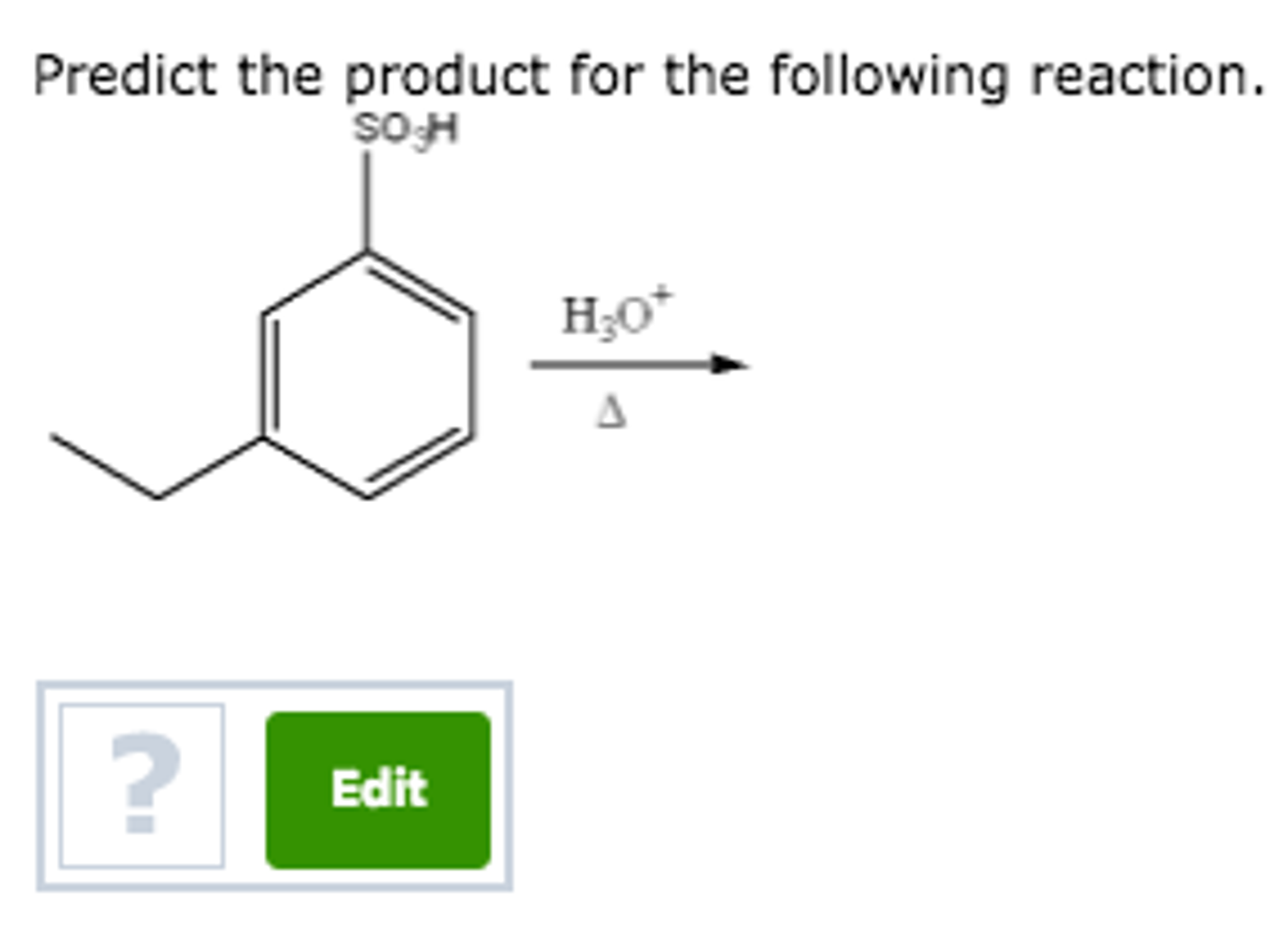
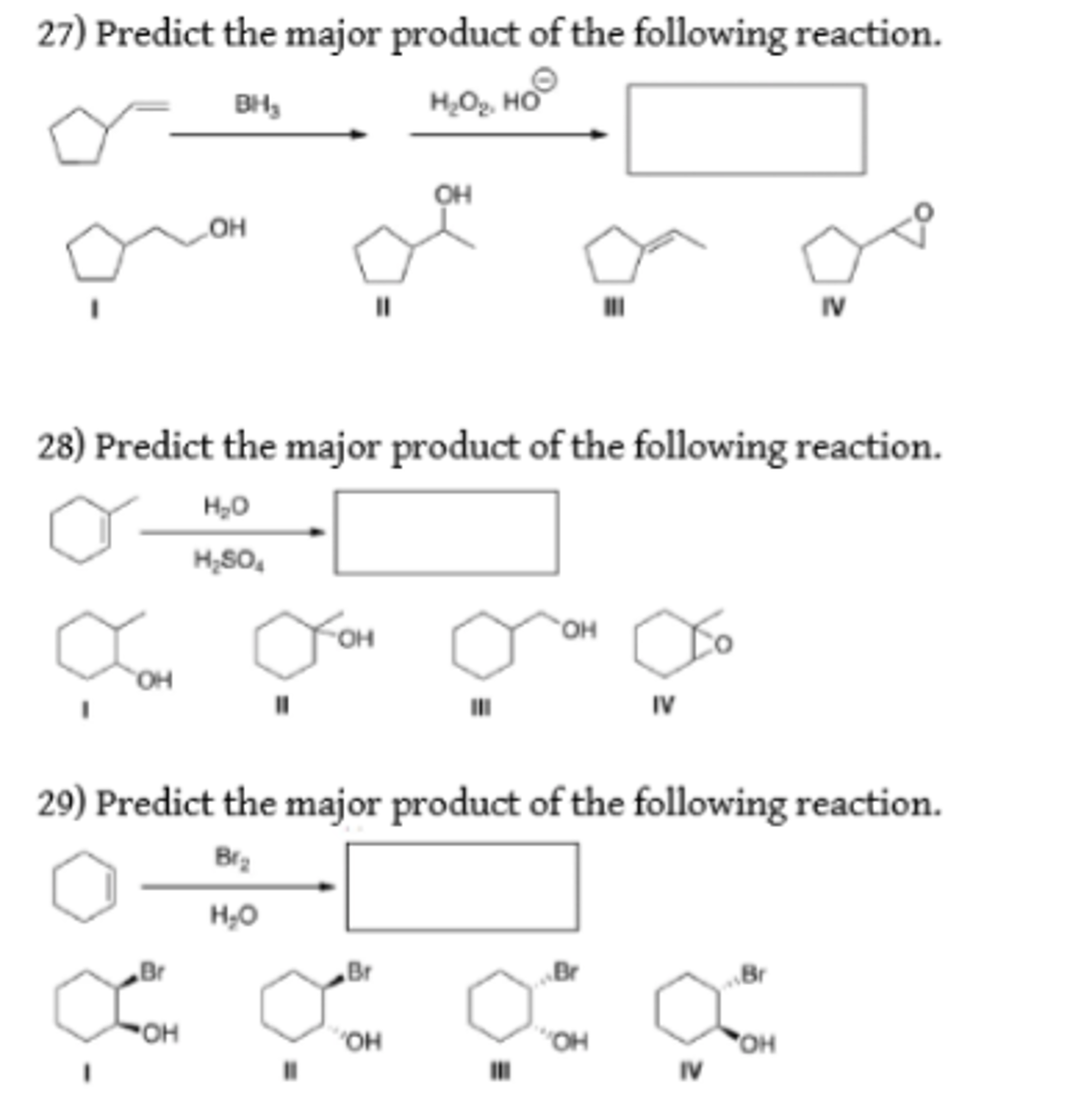
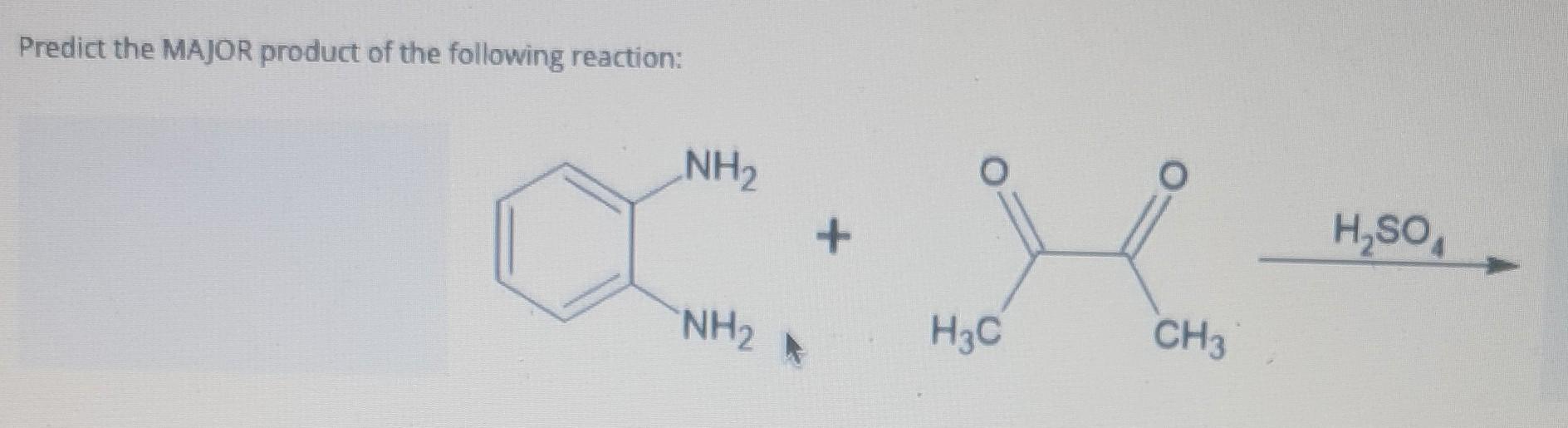
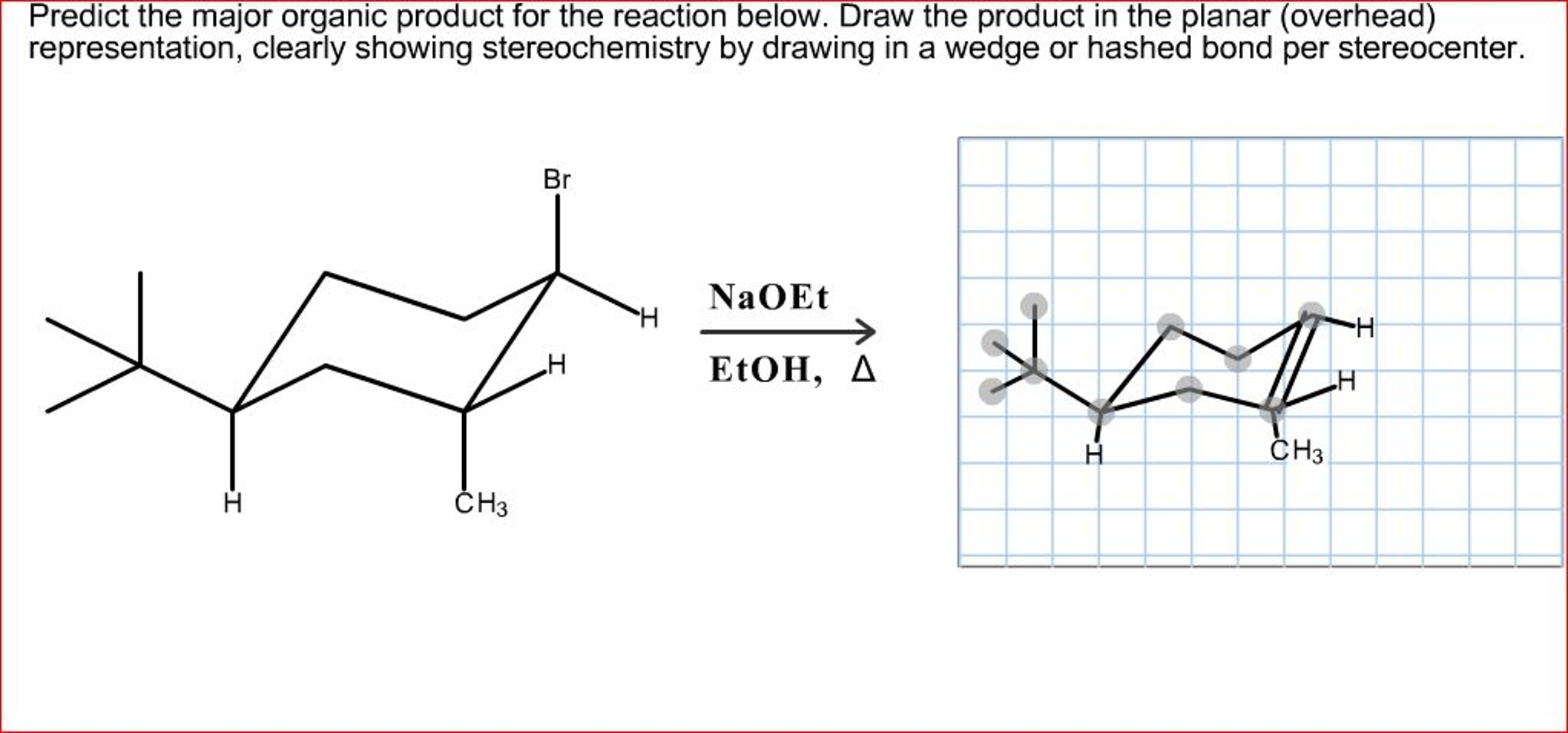
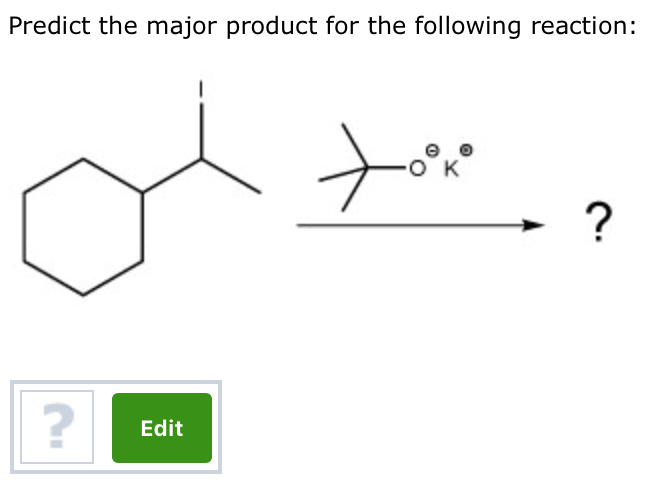
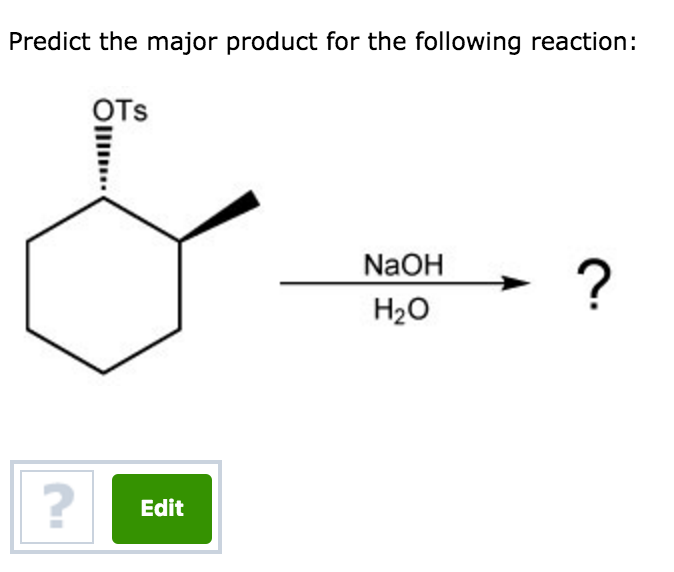
Predict The Major Product For The Reaction.

The seemingly simple query, "Predict the major product for the reaction," echoes through the digital halls of chemistry education and research, sparking debate, confusion, and the occasional flash of brilliance. This deceptively straightforward question, posed daily by students and seasoned researchers alike, demands not just rote memorization but a deep understanding of reaction mechanisms, steric effects, and a host of other chemical principles.
At the heart of this common question lies the challenge of applying theoretical knowledge to predict real-world outcomes. The ability to accurately predict the major product of a chemical reaction is crucial for fields ranging from drug discovery to materials science. It determines the efficiency of syntheses, the potential for unwanted side products, and ultimately, the success of countless chemical endeavors.
The Core Challenge: Mechanism and Prediction
The challenge originates from the inherent complexity of chemical reactions. A reaction pathway, in reality, involves a series of elementary steps, each with its own activation energy and equilibrium constant. Predicting the *major product* requires identifying the pathway with the lowest overall activation energy, leading to the most thermodynamically stable or kinetically favored product.
Consider, for example, a simple electrophilic addition to an alkene. Will the addition follow Markovnikov's rule, or will anti-Markovnikov addition prevail due to specific reaction conditions? This determination hinges on a solid understanding of carbocation stability and the presence of any radical initiators.
Factors Influencing Product Formation
Several key factors influence the product distribution in a chemical reaction. These include the *steric hindrance* around the reaction center, the *electronic effects* of substituents, and the *reaction conditions*, such as temperature, solvent, and the presence of catalysts.
Steric hindrance can prevent bulky groups from approaching the reaction center, favoring a less hindered pathway. Electronic effects, such as inductive and resonance effects, can stabilize or destabilize intermediates, altering the reaction pathway. The solvent can also dramatically impact reactivity by solvating reactants and intermediates differently.
Dr. Anya Sharma, a professor of organic chemistry at MIT, emphasizes the importance of a holistic approach. "Students often focus on memorizing rules, but the key is understanding *why* those rules exist. It's about visualizing the reaction at a molecular level and considering all the factors that might influence the outcome."
The Role of Computational Chemistry
In recent years, computational chemistry has emerged as a powerful tool for predicting reaction outcomes. Density Functional Theory (DFT) and other quantum mechanical methods can accurately model reaction pathways and predict the relative energies of different transition states.
This allows researchers to screen potential reactions in silico, optimizing reaction conditions and minimizing the risk of unwanted side products. Software packages are becoming increasingly accessible, enabling researchers to make informed decisions based on reliable data.
However, even the most sophisticated computational models have limitations. Approximations are inherent in the calculations, and the accuracy of the predictions depends heavily on the quality of the input parameters and the chosen theoretical method. Experimental validation remains crucial.
Educational Approaches and Common Pitfalls
Instructors face the challenge of teaching students to think critically about reaction mechanisms and product prediction. Rote memorization of reaction "rules" often leads to mistakes when faced with unfamiliar or complex reactions.
One common pitfall is failing to consider all possible reaction pathways. Students often focus on the most obvious or intuitive pathway, overlooking alternative mechanisms that might be favored under specific conditions.
Professor David Chen from Stanford University advocates for a problem-based learning approach. "Instead of simply lecturing on reaction mechanisms, we challenge students with complex synthesis problems that require them to apply their knowledge and think creatively. This approach fosters a deeper understanding and improves their ability to predict reaction outcomes."
The Impact on Industry and Research
Accurate product prediction has a profound impact on various industries. In the pharmaceutical industry, it is essential for designing efficient syntheses of drug candidates, minimizing waste, and ensuring the purity of the final product.
In materials science, product prediction guides the development of new polymers, catalysts, and other materials with tailored properties. The ability to predict the outcome of a polymerization reaction, for instance, is crucial for controlling the molecular weight and architecture of the resulting polymer.
Dr. Emily Carter, a leading researcher in computational materials science at Princeton University, notes, "The ability to predict reaction outcomes using computational methods is revolutionizing materials discovery. We can now screen thousands of potential reactions in silico, identifying promising candidates for experimental synthesis and characterization."
Looking Ahead: The Future of Product Prediction
The future of product prediction lies in the integration of computational chemistry, machine learning, and experimental data. Machine learning algorithms can be trained on vast datasets of reaction outcomes, allowing them to identify patterns and predict the products of novel reactions with increasing accuracy.
Furthermore, advances in analytical techniques, such as high-throughput screening and microfluidic reactors, are enabling researchers to rapidly generate experimental data that can be used to validate and refine computational models.
As computational power continues to increase and machine learning algorithms become more sophisticated, the ability to predict the major product of a reaction will become even more accurate and reliable. This will accelerate the pace of discovery in chemistry and related fields, leading to new technologies and solutions to global challenges.