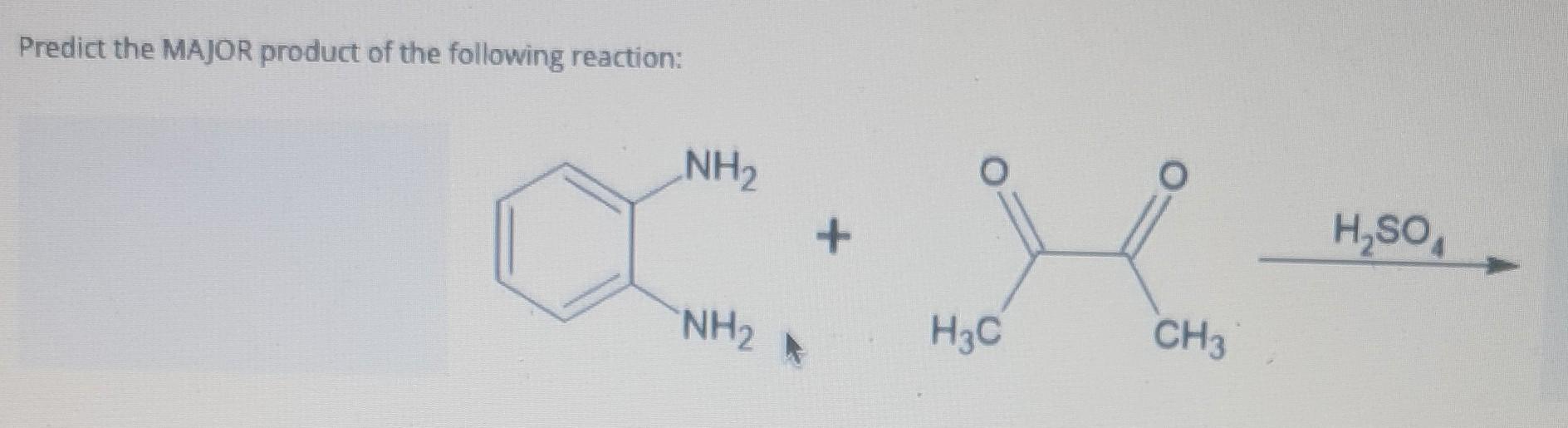
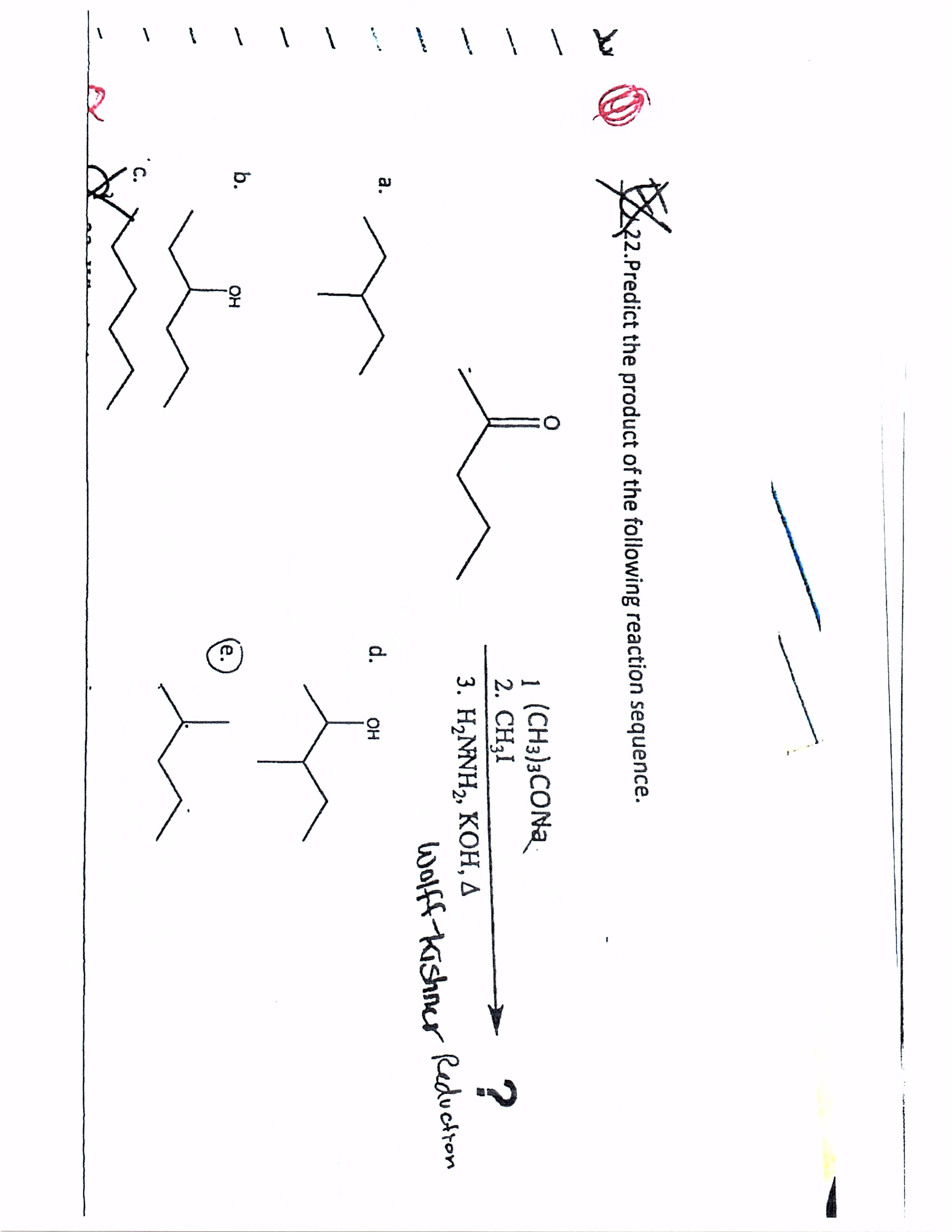
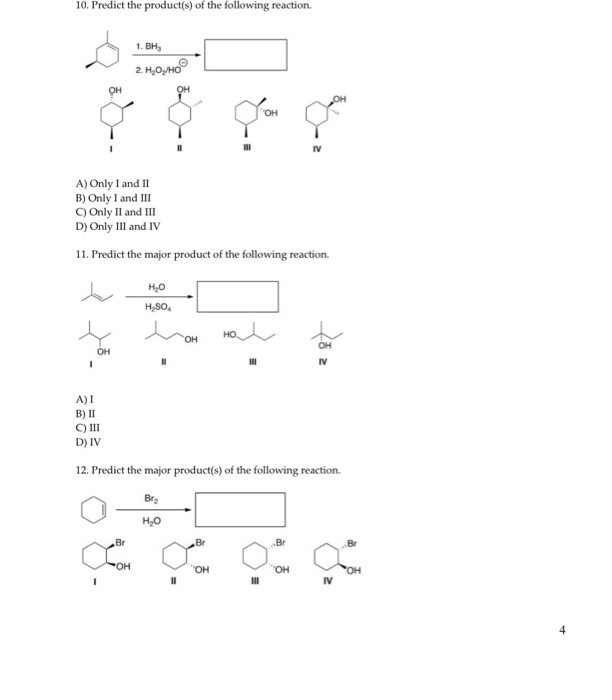
Predict The Product For The Following Reaction
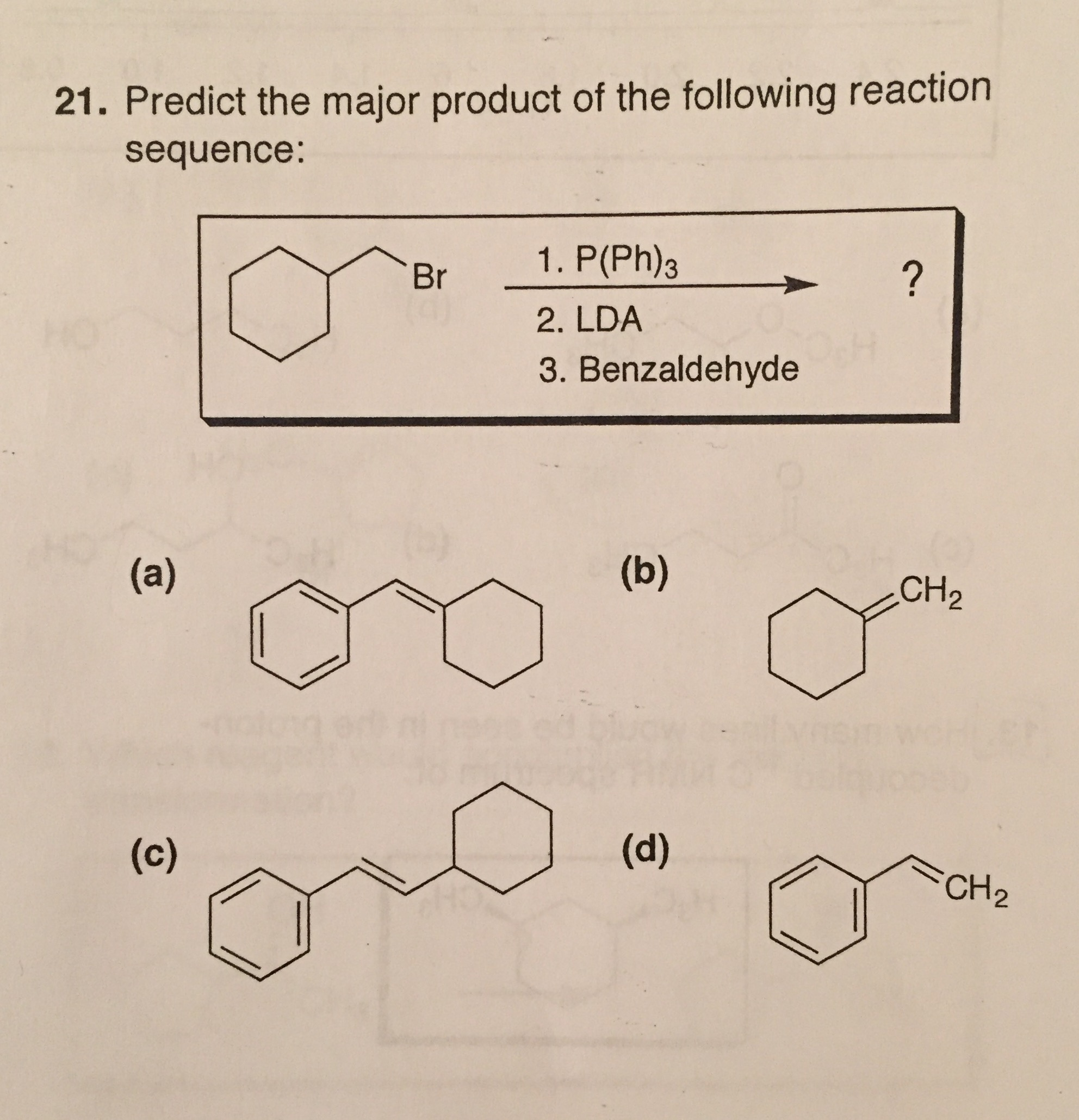
The scientific community is abuzz following a recent theoretical prediction regarding a novel chemical reaction. The potential product, if synthesized successfully, could revolutionize various fields, including materials science and drug development. However, skepticism and challenges remain, casting a shadow of uncertainty over the proposed transformation.
At the heart of the debate lies the complexity of predicting the outcome of a chemical reaction. The so-called 'product prediction challenge' has become a hot topic among chemists. While computational models offer valuable insights, the unpredictable nature of molecular interactions necessitates experimental verification.
The Reaction in Question
The reaction under scrutiny involves a complex organic molecule subjected to specific catalytic conditions. According to the theoretical model, the original molecule will undergo a series of intramolecular rearrangements, forming a highly strained, cage-like structure. This new structure possesses unique electronic properties and potential for further functionalization.
Professor Evelyn Reed from the Massachusetts Institute of Technology (MIT), who led the computational study, published the findings in the prestigious journal Nature Chemistry. Her team utilized density functional theory (DFT) calculations to map out the reaction pathway. This theoretical framework suggests that the reaction is thermodynamically favorable.
Catalyst Specificity
A crucial aspect of the reaction is the role of the catalyst. The theoretical model indicates that a specific ruthenium-based complex is essential for promoting the desired transformation. Alternative catalysts are predicted to lead to different products or no reaction at all.
The selection of this catalyst was informed by its previously demonstrated ability to facilitate similar bond-forming events. The prediction hinges on the catalyst's ability to selectively cleave and form specific bonds within the starting molecule.
Skepticism and Challenges
Despite the compelling theoretical evidence, some researchers express concerns regarding the feasibility of the reaction. The high degree of strain predicted in the product raises questions about its stability.
Professor David Chen, a synthetic chemist at Stanford University, commented, "While the computational results are intriguing, synthesizing such a strained molecule is a formidable challenge. Steric hindrance and competing reaction pathways could easily lead to the formation of undesired byproducts."
Experimental Hurdles
Reproducing the theoretically predicted outcome in the lab presents significant challenges. Precisely controlling reaction conditions, such as temperature, pressure, and solvent, is crucial for achieving the desired selectivity.
The synthesis of the ruthenium-based catalyst itself is a multi-step process, requiring specialized expertise and equipment. Furthermore, isolating and characterizing the product may prove difficult due to its predicted instability.
Potential Applications
If successfully synthesized, the predicted product could have a wide range of applications. Its unique cage-like structure could serve as a building block for novel materials with exceptional mechanical strength.
The molecule's electronic properties may make it suitable for use in organic light-emitting diodes (OLEDs) or as a component in solar cells. Furthermore, the strained structure could be exploited in drug design, enabling the creation of highly specific and potent therapeutic agents.
One potential application lies in targeted drug delivery. The cage-like structure could encapsulate drug molecules, releasing them only at specific locations within the body.
The Next Steps
Several research groups are currently attempting to synthesize the predicted product. Professor Reed's team at MIT is collaborating with experimental chemists to optimize the reaction conditions.
They are exploring various strategies to overcome the synthetic challenges, including the use of protecting groups and flow chemistry techniques. Preliminary results are expected to be published within the next few months.
Professor Chen's lab at Stanford is taking a different approach, focusing on synthesizing simpler analogs of the predicted product to gain a better understanding of its stability and reactivity. Their work aims to provide valuable insights for designing more robust synthetic strategies.
The Future of Product Prediction
The ongoing efforts to synthesize the predicted product highlight the increasing importance of computational chemistry in guiding experimental research. Advances in computational power and algorithms are enabling scientists to predict the outcomes of complex reactions with greater accuracy.
While experimental verification remains essential, computational models can significantly accelerate the discovery process. The integration of computational and experimental approaches is likely to become increasingly prevalent in chemistry research.
The success or failure of this particular product prediction will undoubtedly have a significant impact on the field. It serves as a crucial test case for evaluating the reliability of current theoretical methods. Regardless of the outcome, the pursuit of this challenging reaction is pushing the boundaries of chemical knowledge and innovation. The implications of proving or disproving the prediction will guide future research in both computational and synthetic chemistry, shaping the strategies researchers employ in approaching novel chemical transformations.




![Predict The Product For The Following Reaction Solved [2] Predict the product of the following reaction. 1. | Chegg.com](https://media.cheggcdn.com/media/86c/86c041b2-f348-475b-8518-9568911f193b/image.png)