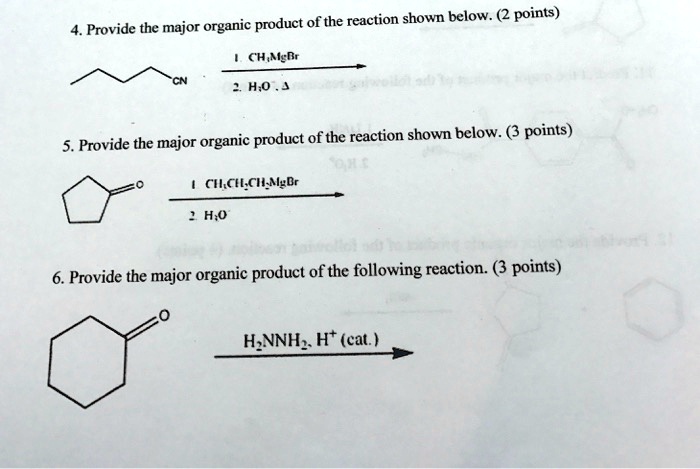
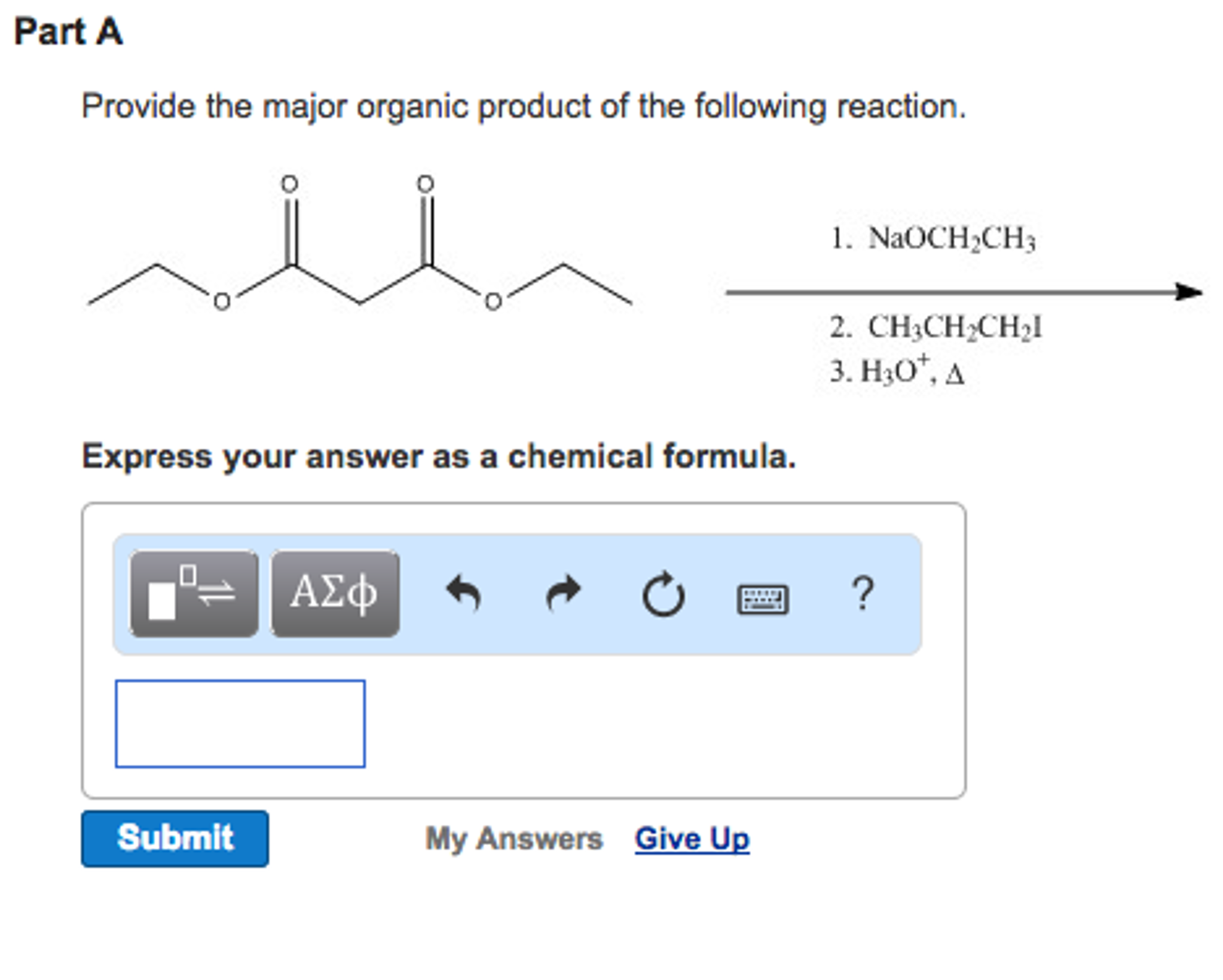
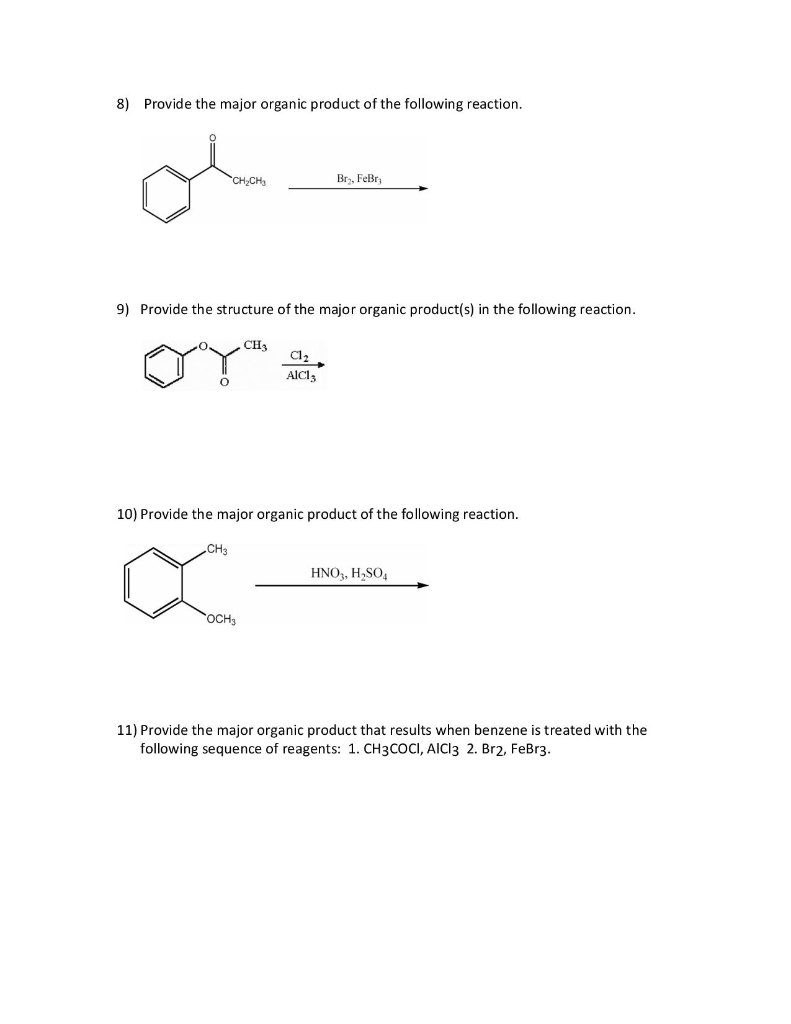
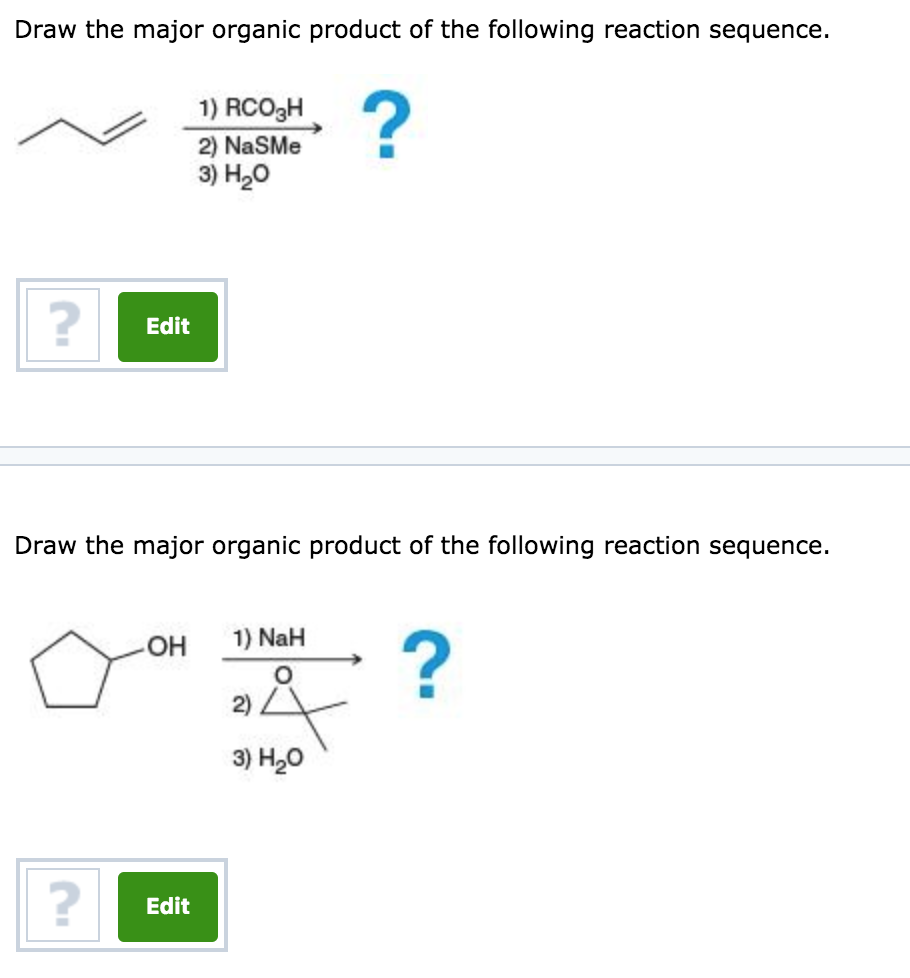
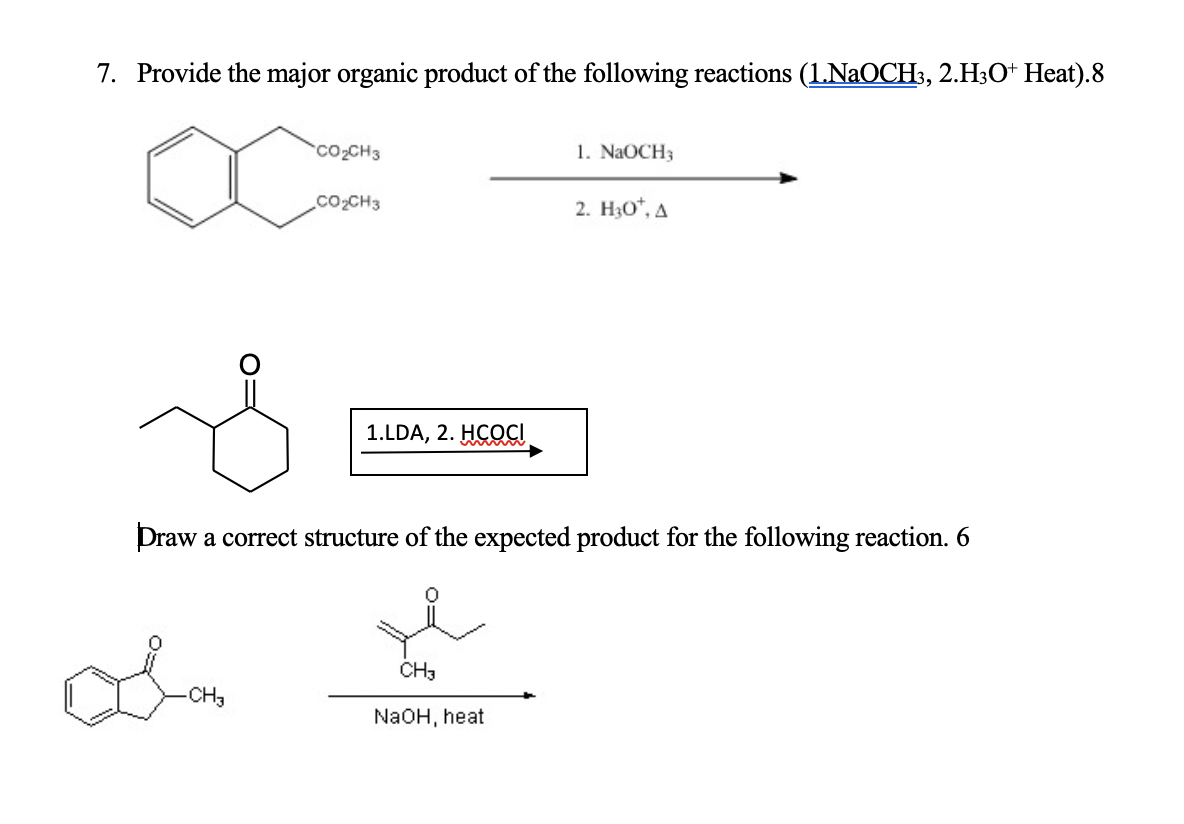
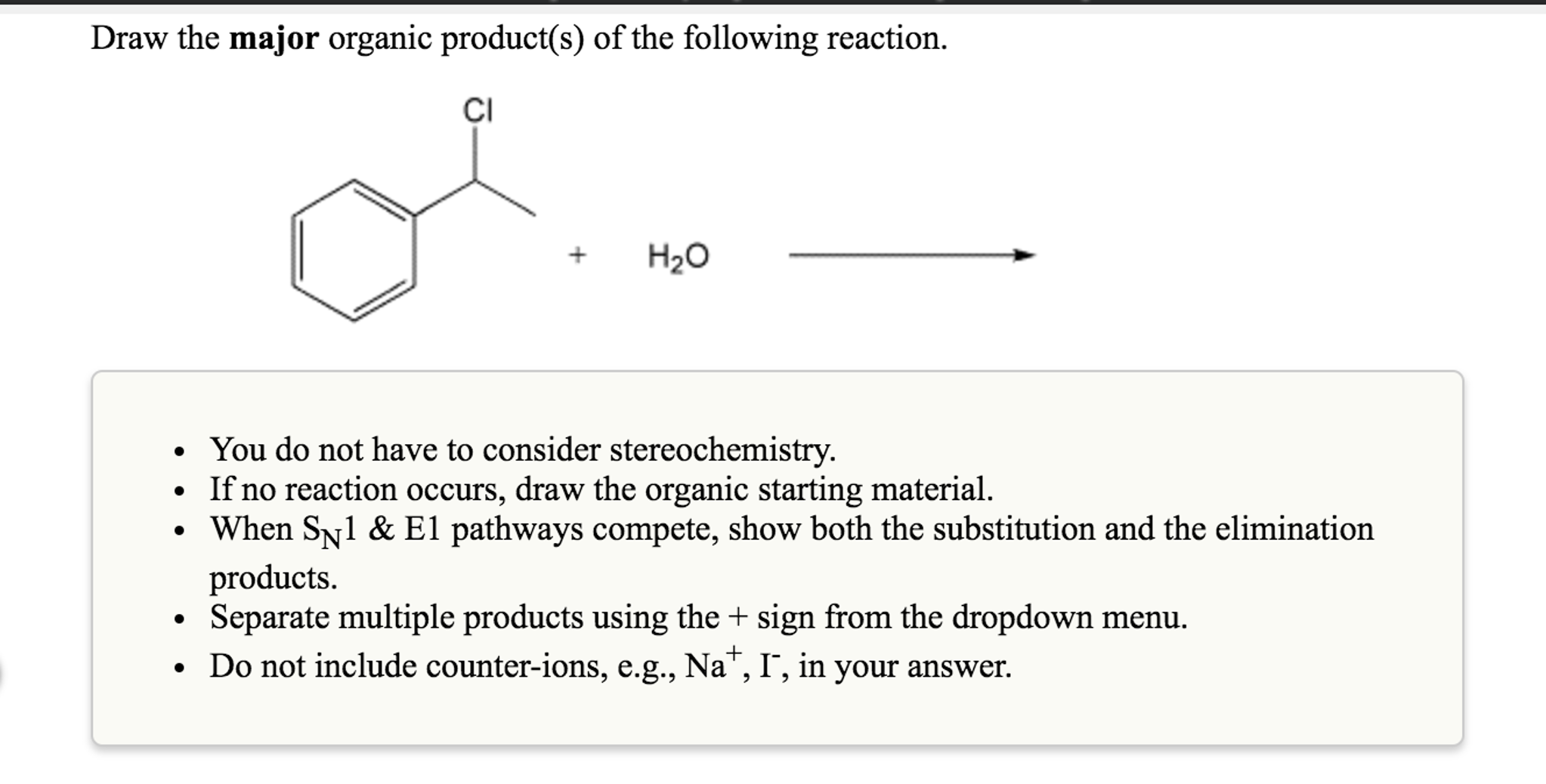
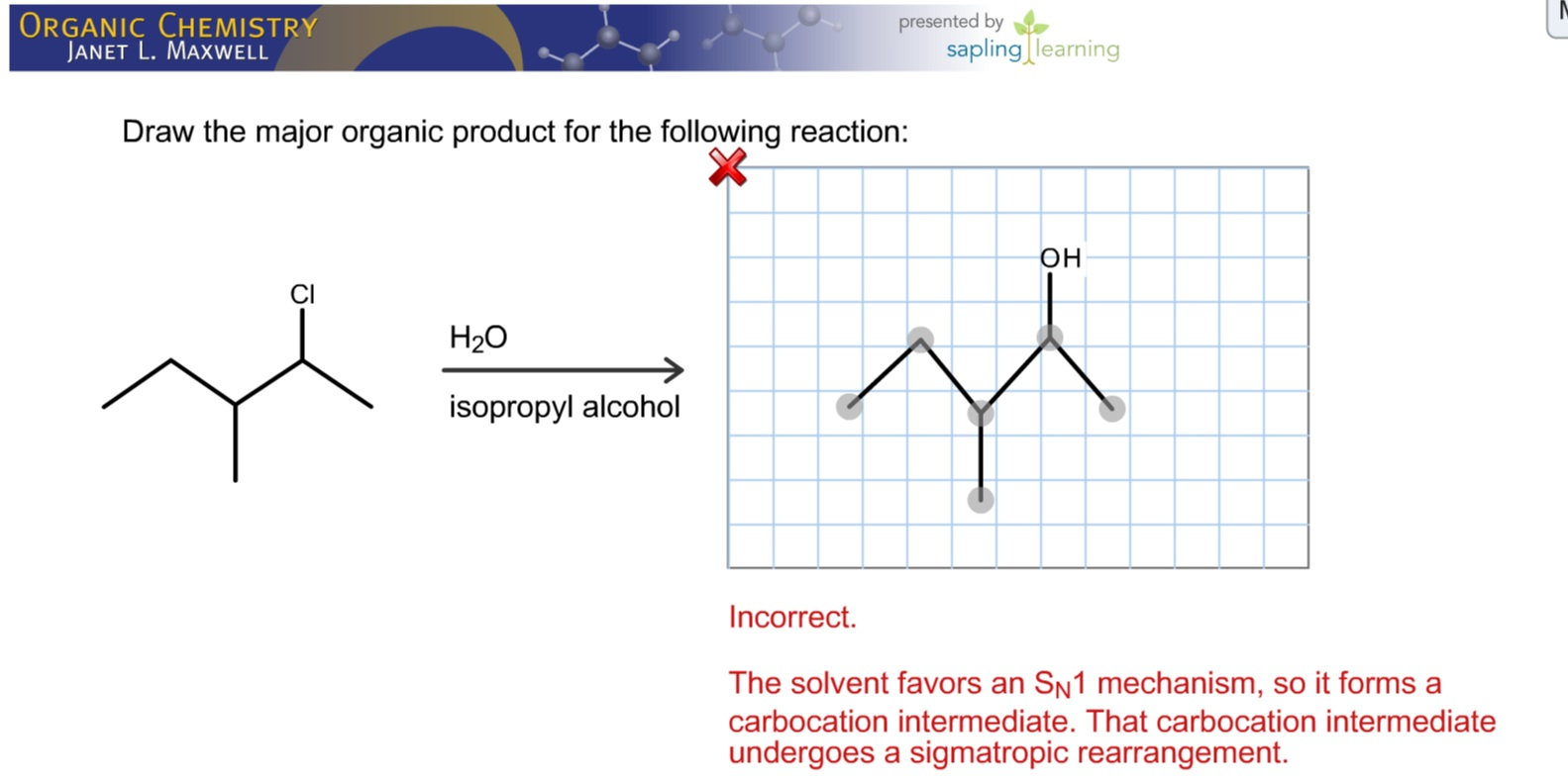
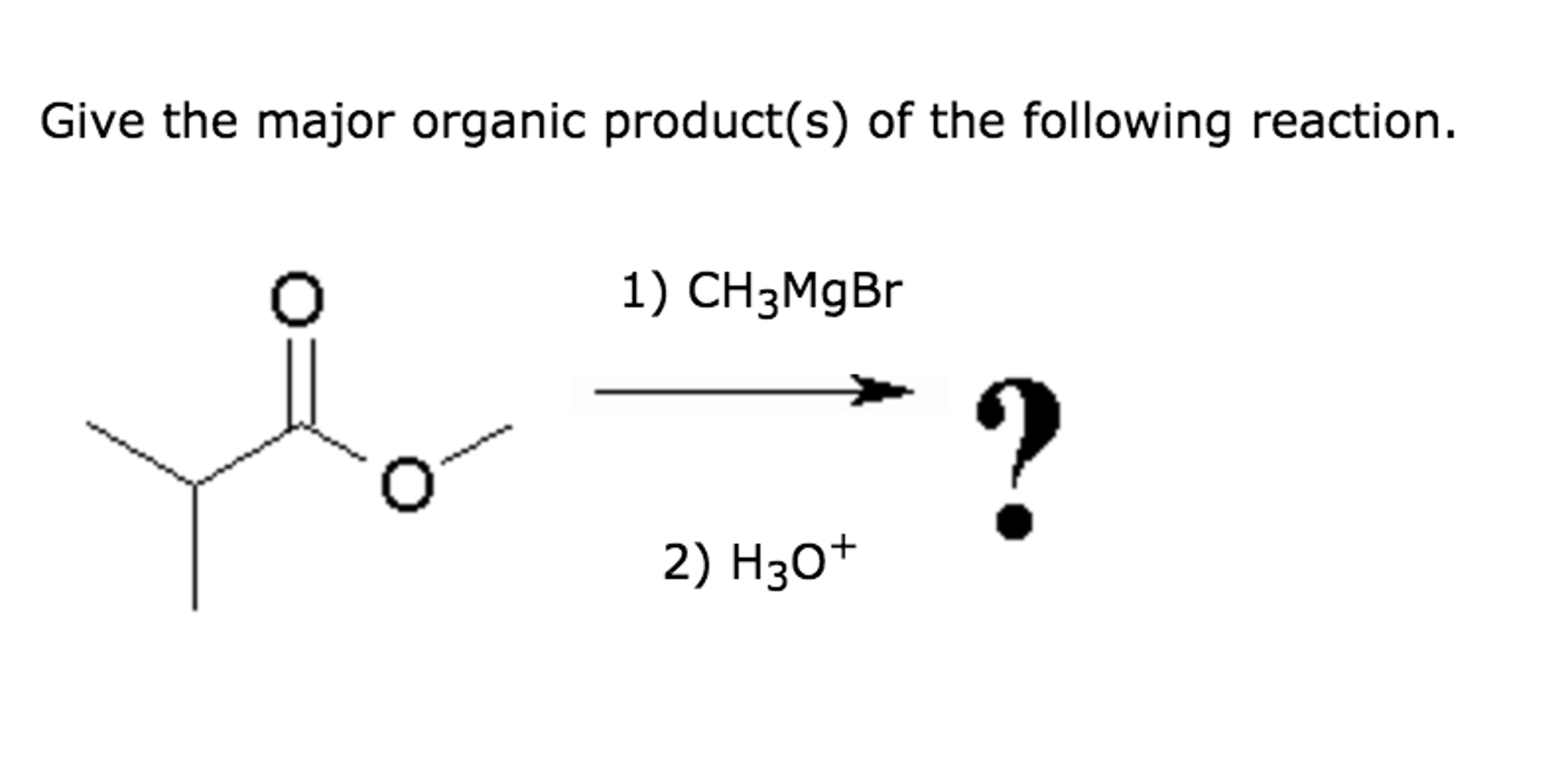
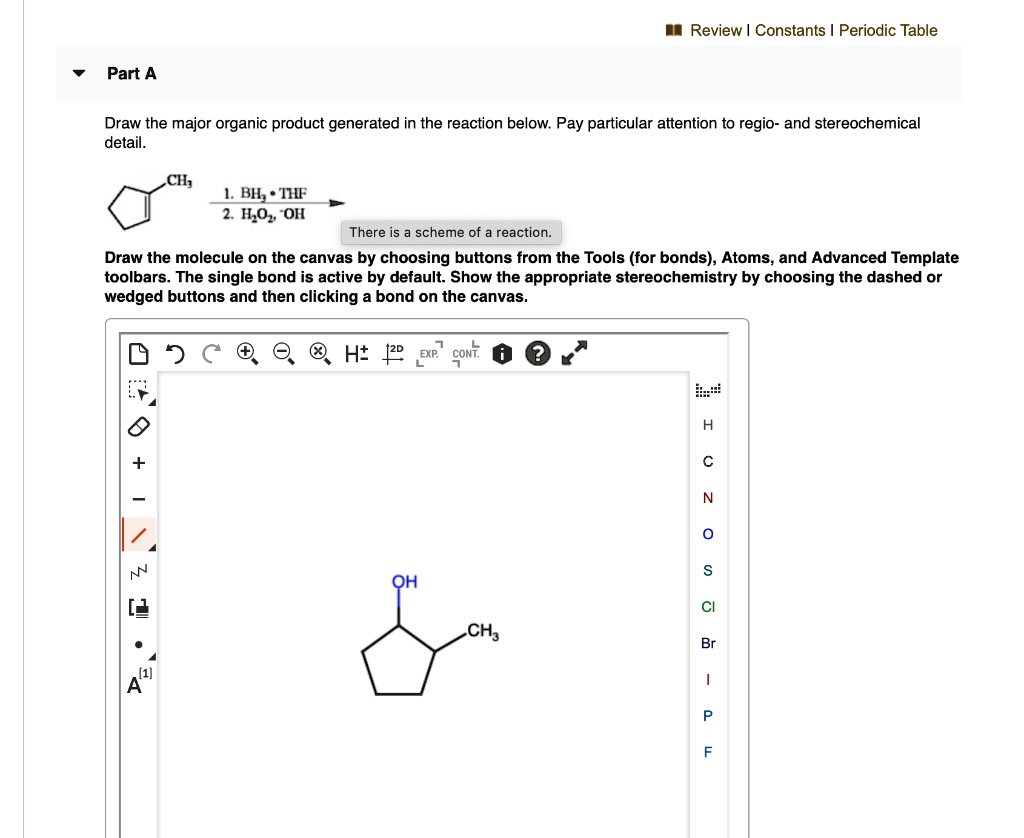
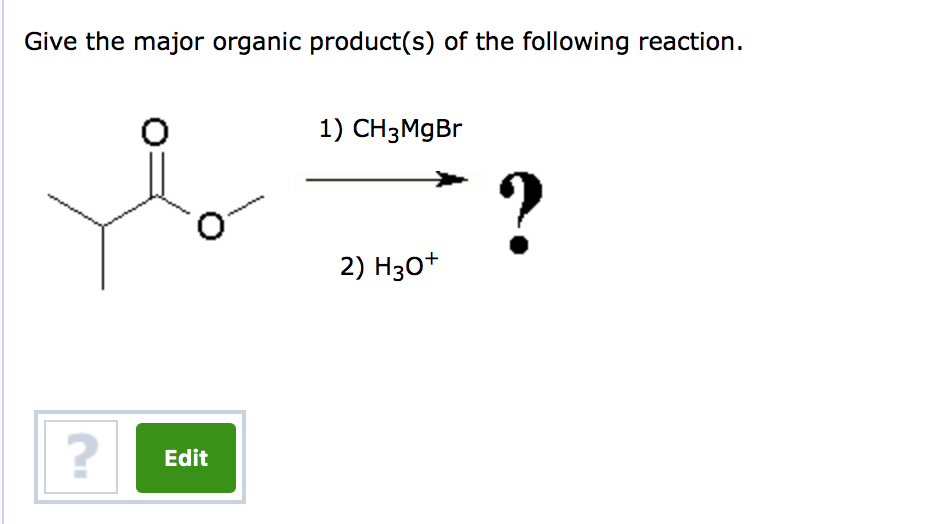
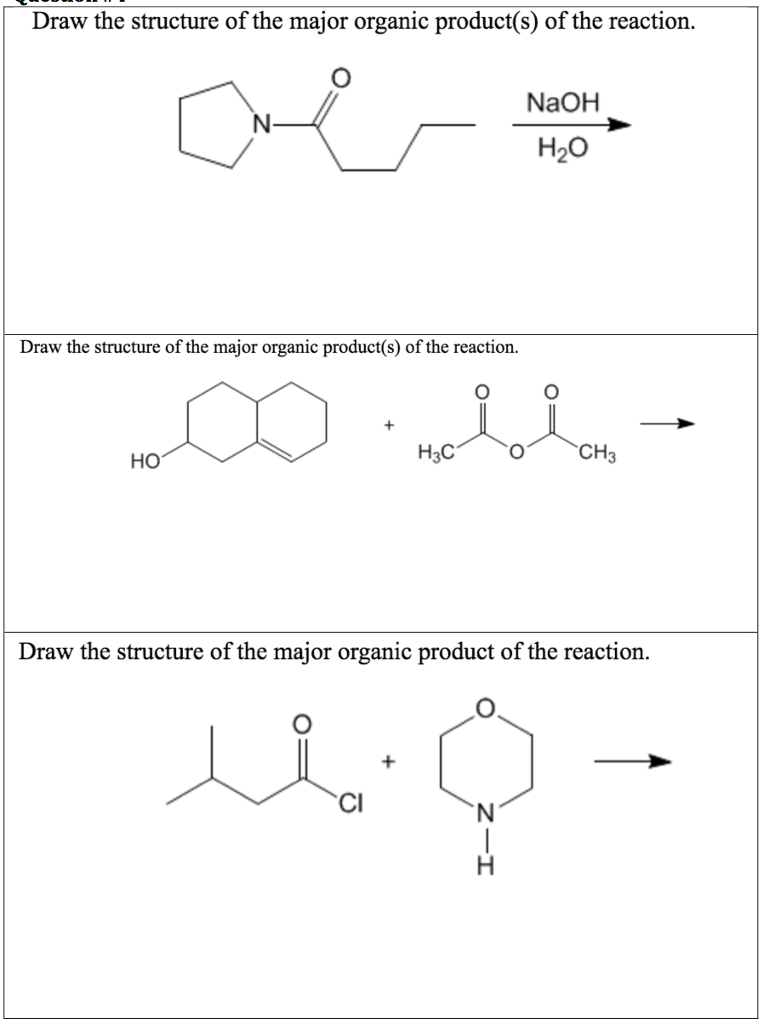
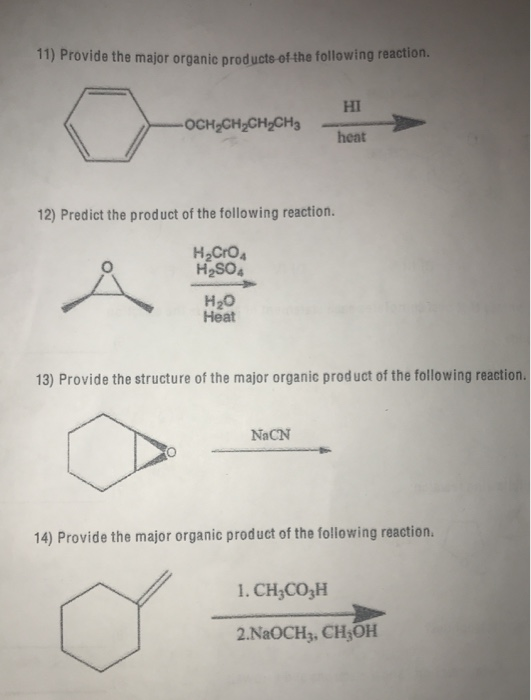
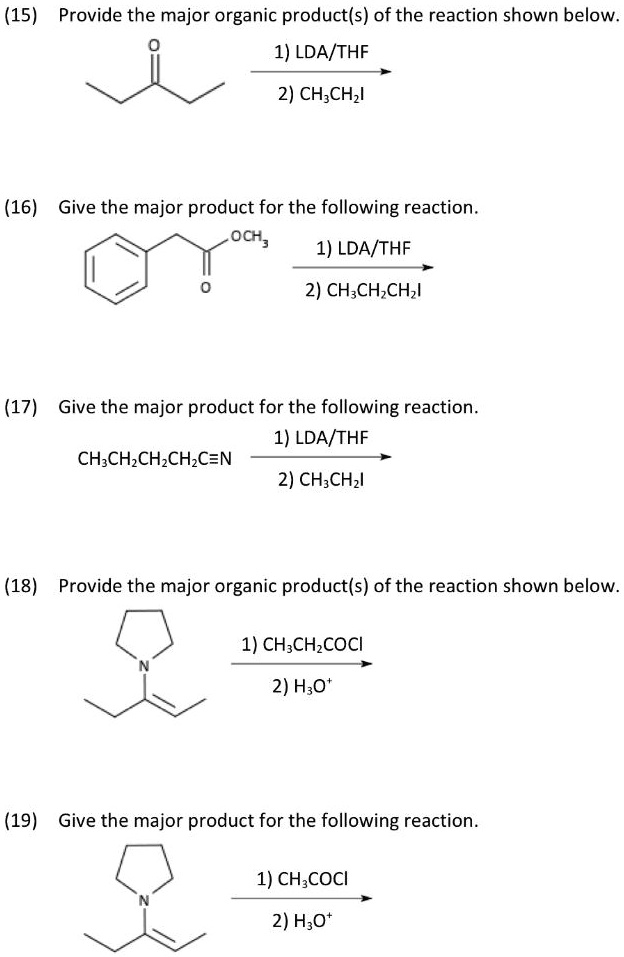
Provide The Major Organic Product Of The Following
Imagine a world where complex organic reactions are not shrouded in mystery but unveiled with clarity, where the journey from reactants to products becomes a predictable path. Organic chemistry, often perceived as a daunting subject, holds the key to understanding the very molecules that make up our world.
This article will explore some major organic reactions, identifying the principal organic product formed in each case. We will focus on simplicity and clarity, aiming to provide a useful guide for those delving into the realm of organic chemistry.
Understanding Organic Reactions
Organic reactions are the fundamental processes that drive the synthesis of new molecules in organic chemistry. Predicting the major organic product of a given reaction is a core skill for chemists.
The "major" product is the organic compound formed in the greatest amount, favored by the reaction conditions and mechanism.
Esterification
Esterification is the reaction between a carboxylic acid and an alcohol, typically catalyzed by an acid.
The major organic product is an ester and water.
For example, the reaction of acetic acid (CH3COOH) with ethanol (CH3CH2OH) in the presence of sulfuric acid (H2SO4) yields ethyl acetate (CH3COOCH2CH3) as the major product.
Saponification
Saponification is the alkaline hydrolysis of a fat or oil, converting it into soap and glycerol.
The major organic products are soap (a salt of a fatty acid) and glycerol.
For instance, the saponification of triglycerides with sodium hydroxide (NaOH) generates glycerol and sodium salts of fatty acids (soap).
Addition Reactions to Alkenes
Alkenes, characterized by their carbon-carbon double bond, undergo addition reactions with a variety of reagents.
The major organic product depends on the specific reagent, but general examples include halides, hydrogen, and water.
Hydrogenation, the addition of hydrogen (H2) to an alkene in the presence of a metal catalyst (like Pt, Pd, or Ni), produces an alkane.
Halogenation, the addition of a halogen (like Cl2 or Br2) to an alkene, yields a vicinal dihalide.
Hydration, the addition of water (H2O) to an alkene in the presence of an acid catalyst, produces an alcohol, following Markovnikov's rule.
Elimination Reactions
Elimination reactions involve the removal of atoms or groups of atoms from a molecule, often leading to the formation of a double or triple bond.
The major organic product is typically an alkene or an alkyne.
For example, the dehydration of an alcohol with concentrated sulfuric acid (H2SO4) at high temperatures generates an alkene.
SN1 and SN2 Reactions
SN1 and SN2 reactions are two fundamental types of nucleophilic substitution reactions in organic chemistry.
In an SN2 reaction, a nucleophile attacks a substrate, displacing a leaving group in a single, concerted step, producing an inverted stereocenter.
In an SN1 reaction, a leaving group departs first, forming a carbocation intermediate. This carbocation is then attacked by a nucleophile. The major organic product is the substituted compound, often with a racemic mixture at the reaction center.
Aldol Condensation
The Aldol condensation is a reaction between two carbonyl compounds (aldehydes or ketones) to form a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give an α,β-unsaturated carbonyl compound.
The major organic product is an α,β-unsaturated aldehyde or ketone.
For example, two molecules of acetaldehyde (CH3CHO) can react under basic conditions to form crotonaldehyde (CH3CH=CHCHO).
Grignard Reactions
Grignard reactions involve the reaction of a Grignard reagent (RMgX) with a carbonyl compound, such as an aldehyde or ketone.
The major organic product, after hydrolysis (addition of water or acid), is an alcohol.
The reaction of a Grignard reagent with an aldehyde produces a secondary alcohol, while the reaction with a ketone produces a tertiary alcohol.
Diels-Alder Reaction
The Diels-Alder reaction is a cycloaddition reaction between a conjugated diene and a dienophile (an alkene or alkyne), forming a cyclic compound.
The major organic product is a substituted cyclohexene.
This reaction is highly stereospecific and is widely used in organic synthesis to create complex ring systems.
Oxidation Reactions
Oxidation reactions involve the increase in the oxidation state of a molecule.
The major organic product depends on the oxidizing agent and the starting material.
For example, the oxidation of a primary alcohol can yield either an aldehyde (using PCC) or a carboxylic acid (using KMnO4 or CrO3).
The oxidation of a secondary alcohol yields a ketone.
Reduction Reactions
Reduction reactions involve the decrease in the oxidation state of a molecule.
The major organic product depends on the reducing agent and the starting material.
For instance, the reduction of an aldehyde or ketone with NaBH4 yields an alcohol.
The reduction of a carboxylic acid or ester with LiAlH4 also yields an alcohol.
Significance and Applications
Understanding these major organic products is crucial in various fields, including pharmaceuticals, materials science, and agriculture.
The ability to predict and control organic reactions allows chemists to synthesize complex molecules with desired properties and functions.
The synthesis of pharmaceuticals relies heavily on the precise control of organic reactions to create drugs that target specific biological pathways.
In materials science, organic reactions are used to create polymers and other materials with tailored properties for applications ranging from electronics to construction.
In agriculture, organic reactions play a role in the synthesis of pesticides and herbicides, helping to protect crops and increase yields.
Conclusion
As we journey through the vast landscape of organic chemistry, identifying the major organic product in a reaction is a guiding light.
By mastering these fundamental reactions, we gain a deeper understanding of the intricate molecular world and the power to create new and innovative materials.
The world of organic chemistry holds endless possibilities, inviting us to explore, innovate, and transform the very fabric of our existence.