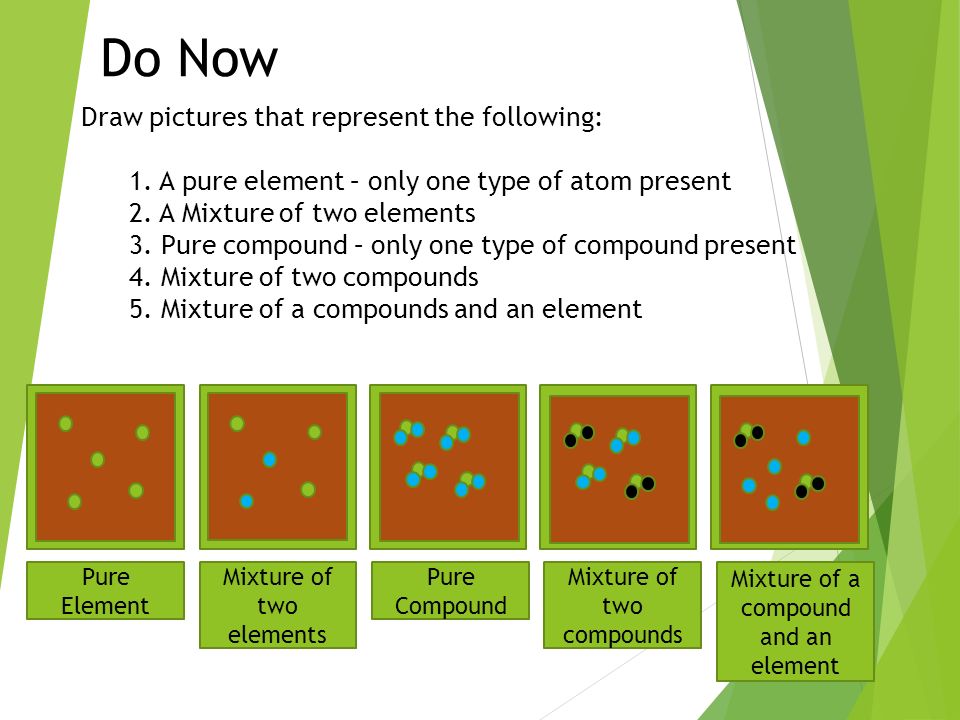
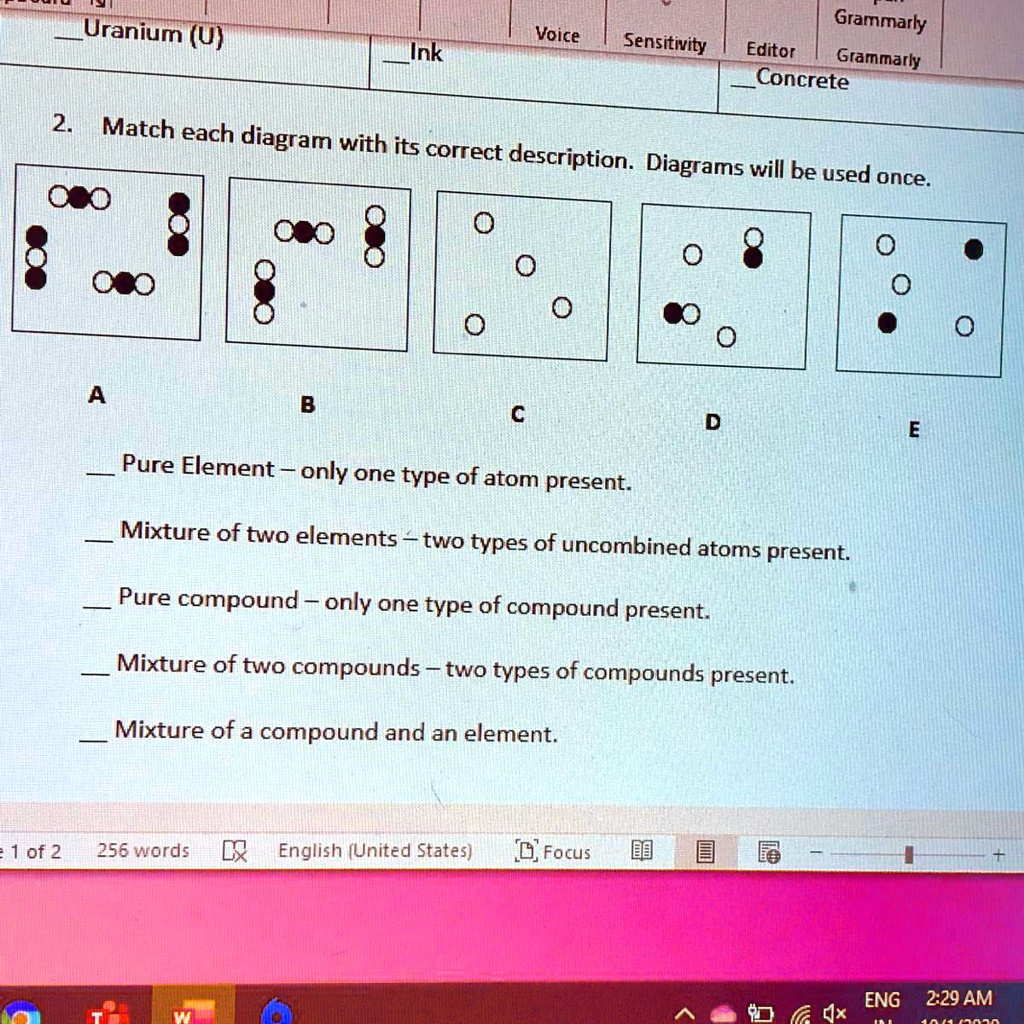
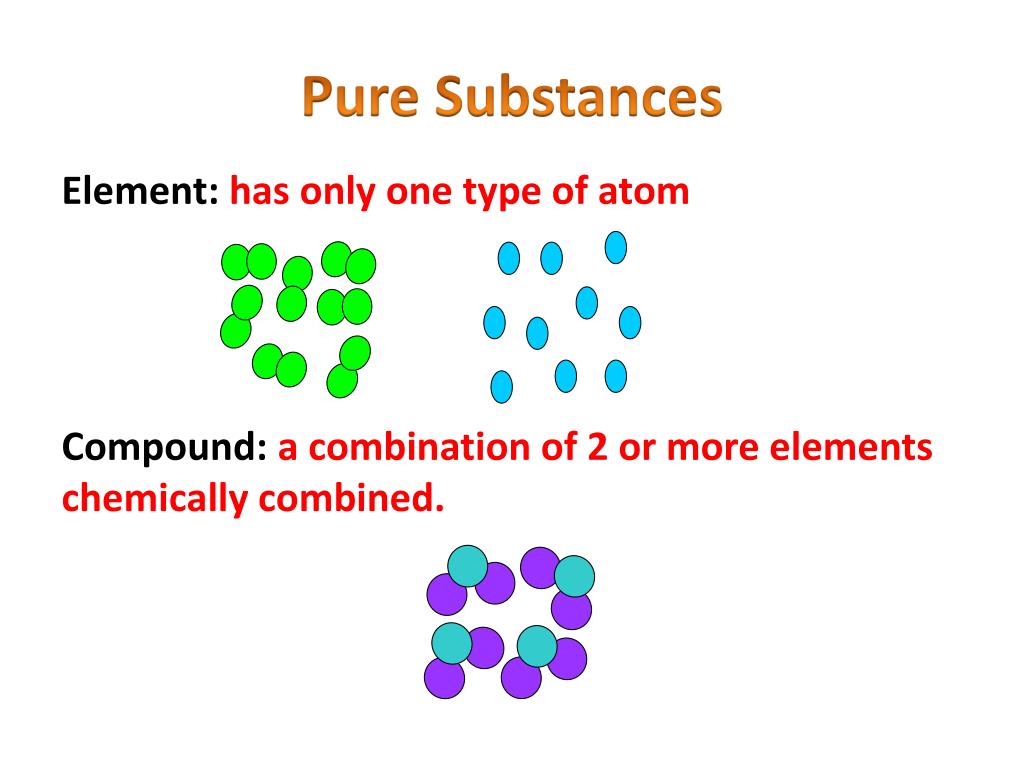
Pure Element Only One Type Of Atom Present

Imagine holding a shimmering gold ring, the sunlight dancing across its surface. Feel the weight of it in your hand, the smooth, cool metal a tangible piece of the earth. What if I told you that this simple, elegant object, at its very core, is made of only one thing: gold atoms, nothing more, nothing less.
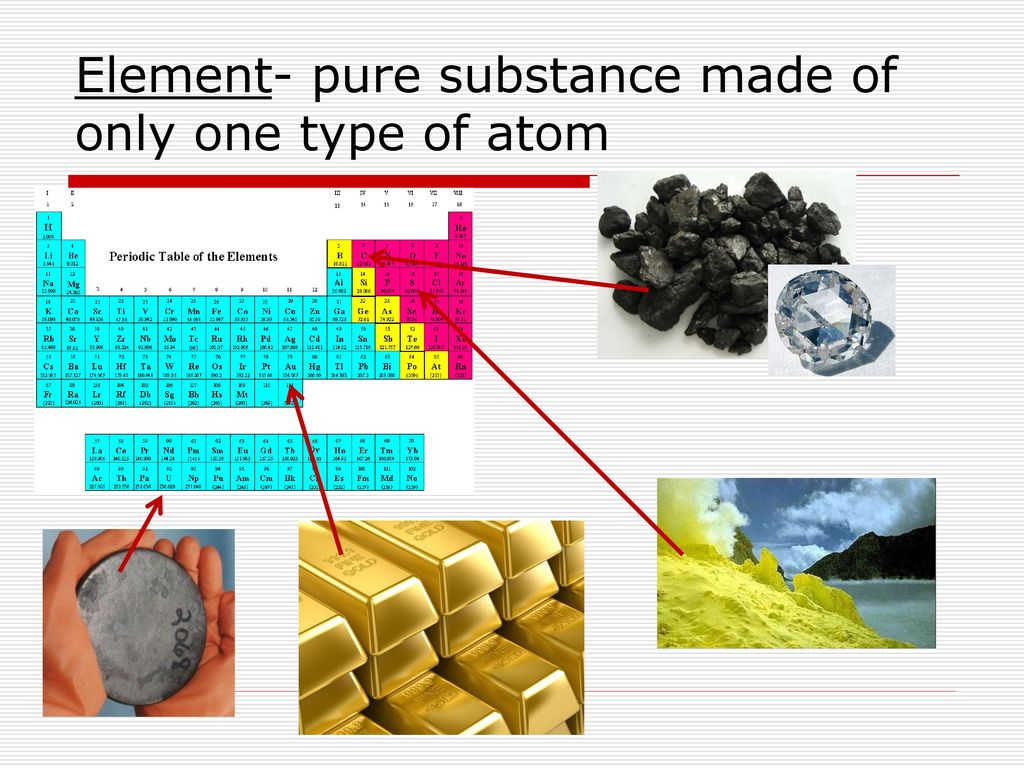
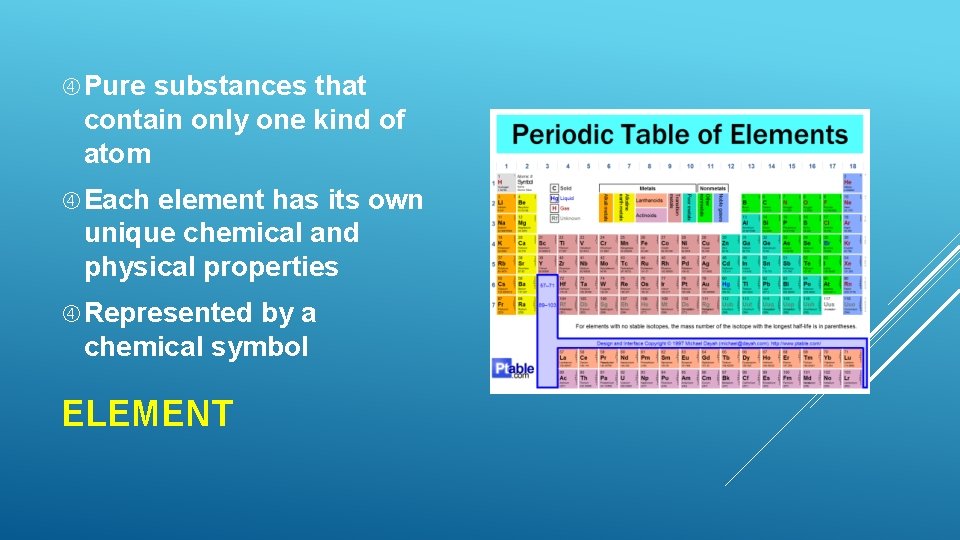
At the heart of chemistry lies a fundamental concept: the pure element. Defined as a substance composed of only one type of atom, pure elements are the building blocks of everything around us, from the air we breathe to the stars in the night sky. This article delves into the significance of pure elements, exploring their properties, history, and ongoing impact on scientific advancement and our everyday lives.
The Essence of Purity: Defining Pure Elements
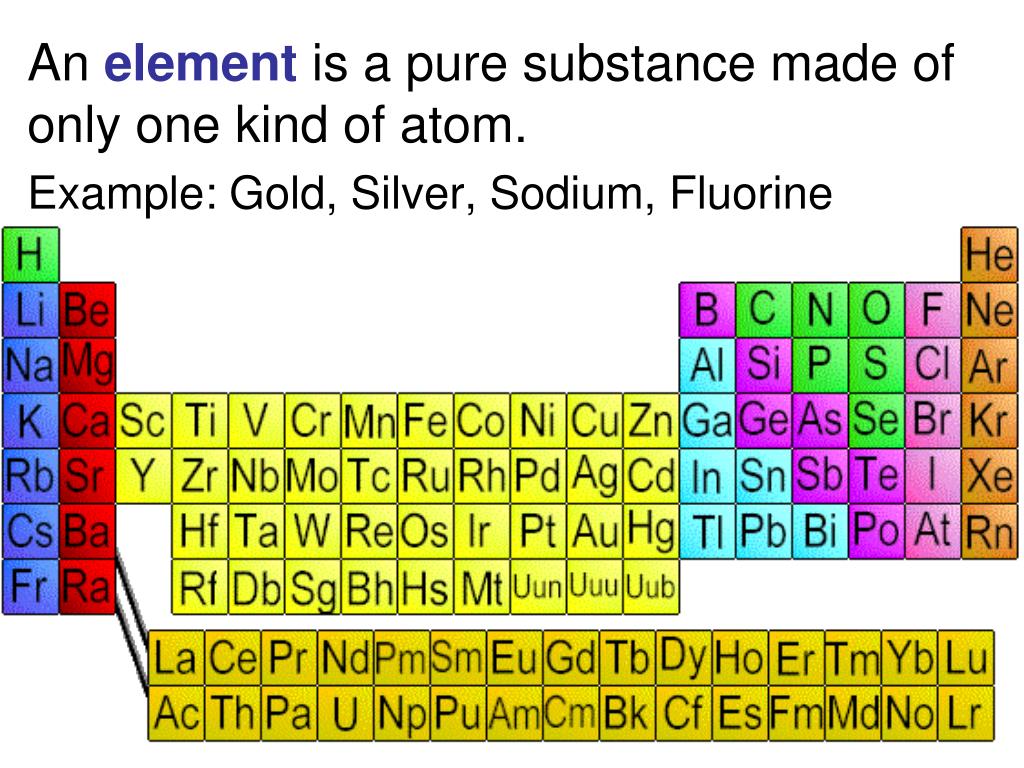
A pure element is the simplest form of matter that cannot be broken down into simpler substances by chemical means. It is defined by its atomic number, which is the number of protons in the nucleus of its atoms. For example, all atoms with 79 protons are gold atoms, and therefore, pure gold consists only of gold atoms.
Unlike compounds, which are formed from two or more different elements chemically bonded together, a pure element exists as a single, uniform substance. This inherent simplicity gives pure elements distinct properties, such as unique melting and boiling points, densities, and reactivity.
A Journey Through the Periodic Table
The periodic table of elements, first organized by Dmitri Mendeleev in 1869, is the cornerstone of chemistry. It neatly arranges all known elements based on their atomic number and recurring chemical properties. Each square on the table represents a pure element, and it's where scientists can find detailed information about each one, including its symbol, atomic mass, and electron configuration.
Elements are broadly classified as metals, nonmetals, and metalloids. Metals, like iron, copper, and aluminum, are typically shiny, malleable, and good conductors of electricity. Nonmetals, such as oxygen, nitrogen, and sulfur, are often gases or brittle solids and poor conductors. Metalloids, like silicon and germanium, exhibit properties of both metals and nonmetals and are vital in semiconductor technology.
Historical Roots: From Alchemy to Modern Chemistry
The pursuit of pure elements has a rich history, stretching back to the days of alchemy. Alchemists sought to transmute base metals into gold, believing that all matter was composed of varying proportions of four fundamental elements: earth, air, fire, and water. While their efforts at transmutation ultimately failed, they developed essential laboratory techniques and laid the groundwork for modern chemistry.
As scientific understanding grew, the definition and identification of pure elements became more precise. The development of atomic theory and the discovery of subatomic particles in the 20th century revolutionized our understanding of matter. Scientists like John Dalton and Ernest Rutherford were instrumental in developing the atomic theory.
Isotopes: Variations on a Theme
While a pure element consists of only one type of atom, it's important to remember the existence of isotopes. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. For example, carbon-12 and carbon-14 are both isotopes of carbon, with carbon-14 containing two more neutrons than carbon-12.
Isotopes have important applications in various fields, including medicine, archaeology, and environmental science. Radioactive isotopes, for instance, are used in medical imaging and cancer therapy, while carbon-14 dating helps determine the age of ancient artifacts.
The Impact of Pure Elements on Technology
Pure elements are indispensable in modern technology. Silicon, in its highly purified form, is the backbone of the semiconductor industry, used to manufacture microchips and electronic devices. Rare earth elements, a group of 17 metallic elements, are crucial components in smartphones, electric vehicles, and renewable energy technologies.
The demand for pure elements has driven advancements in materials science and engineering. Scientists are constantly developing new methods to extract and purify elements with greater efficiency and sustainability. Finding new sources for these elements, particularly rare earth elements, is becoming more and more important for global tech sustainability.
Purity in Our Daily Lives
We encounter pure elements in various aspects of our daily lives, often without even realizing it. The oxygen we breathe, though part of a mixture of gases, is itself a pure element, essential for respiration. The aluminum foil we use to wrap food is made of pure aluminum, a lightweight and corrosion-resistant metal.
Even the water we drink, though typically containing dissolved minerals, is ultimately composed of hydrogen and oxygen, both pure elements. The purity of certain elements is vital in ensuring product safety and effectiveness, especially in medical and pharmaceutical applications.
Challenges and Future Directions
Despite their fundamental nature, obtaining truly pure elements can be challenging. Trace impurities can significantly affect the properties of an element, particularly in sensitive applications. Therefore, sophisticated purification techniques are necessary to achieve the desired level of purity.
Scientists are continuously exploring new methods for synthesizing and purifying elements, as well as discovering new applications for them. The search for new elements with unique properties is an ongoing endeavor, pushing the boundaries of chemical knowledge. The creation of new elements by scientists, albeit fleeting and unstable, expands our understanding of nuclear physics and the limits of matter.
A Final Reflection
The concept of a pure element, seemingly simple at first glance, reveals a profound understanding of the fundamental building blocks of our universe. From the shimmering gold ring to the silicon chip powering your computer, pure elements play a critical role in shaping our world.
As we continue to explore the mysteries of the elements, it is likely that future advancements will uncover even greater potential in these fundamental substances. This continuing discovery and innovations will continue to improve the quality of lives.