Resting Membrane Potential Of Skeletal Muscle
The foundation of muscle contraction, enabling everything from a gentle blink to a powerful sprint, lies in a finely tuned electrical property: the resting membrane potential of skeletal muscle. This potential, a state of electrical disequilibrium across the muscle cell membrane, is crucial for initiating the cascade of events that leads to muscle fiber activation.
Understanding this fundamental concept is vital not only for biologists and medical professionals but also for athletes, trainers, and anyone interested in the intricacies of human movement. Disruptions to the resting membrane potential can lead to various neuromuscular disorders, highlighting its clinical significance.
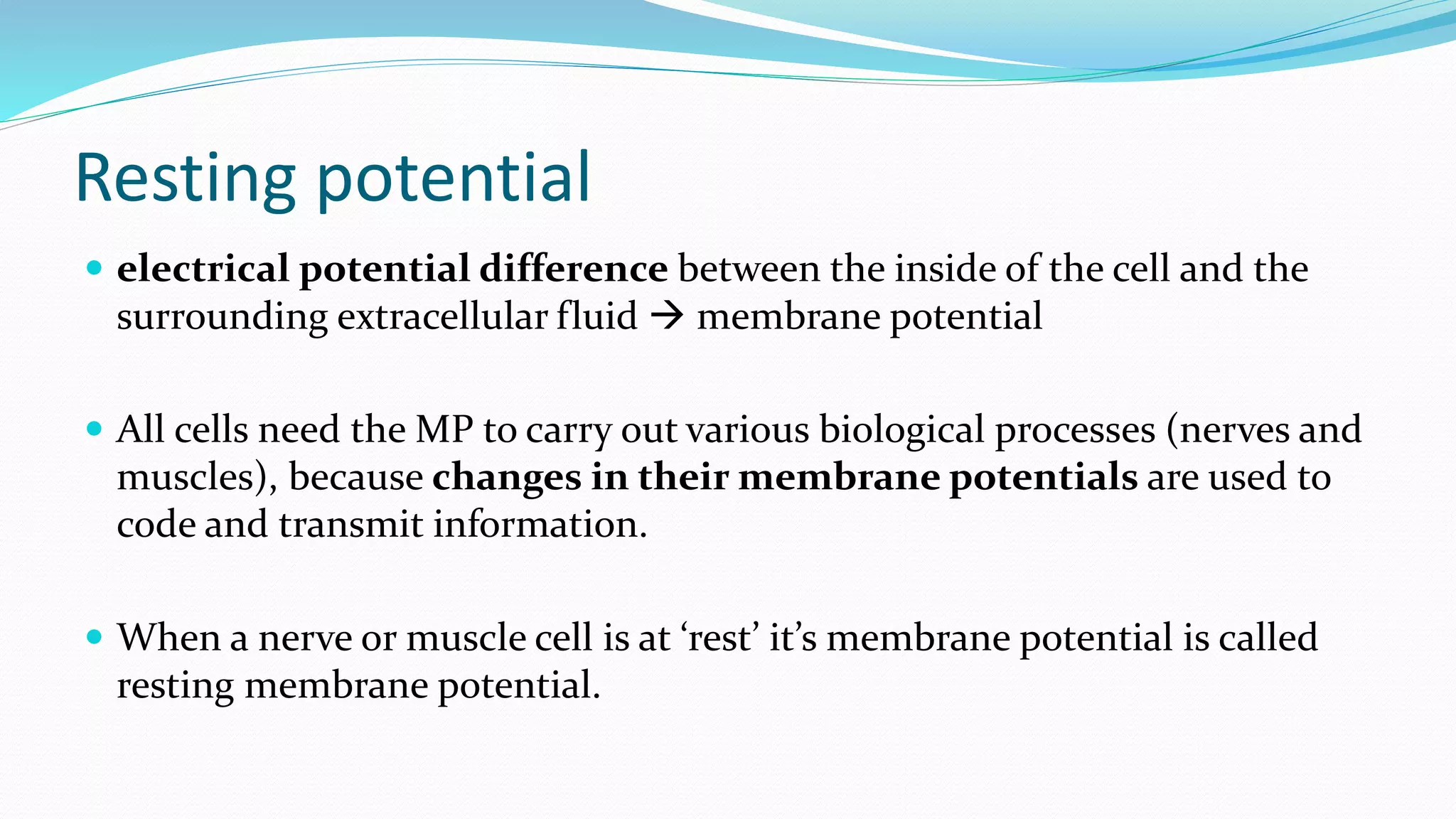
What is Resting Membrane Potential?
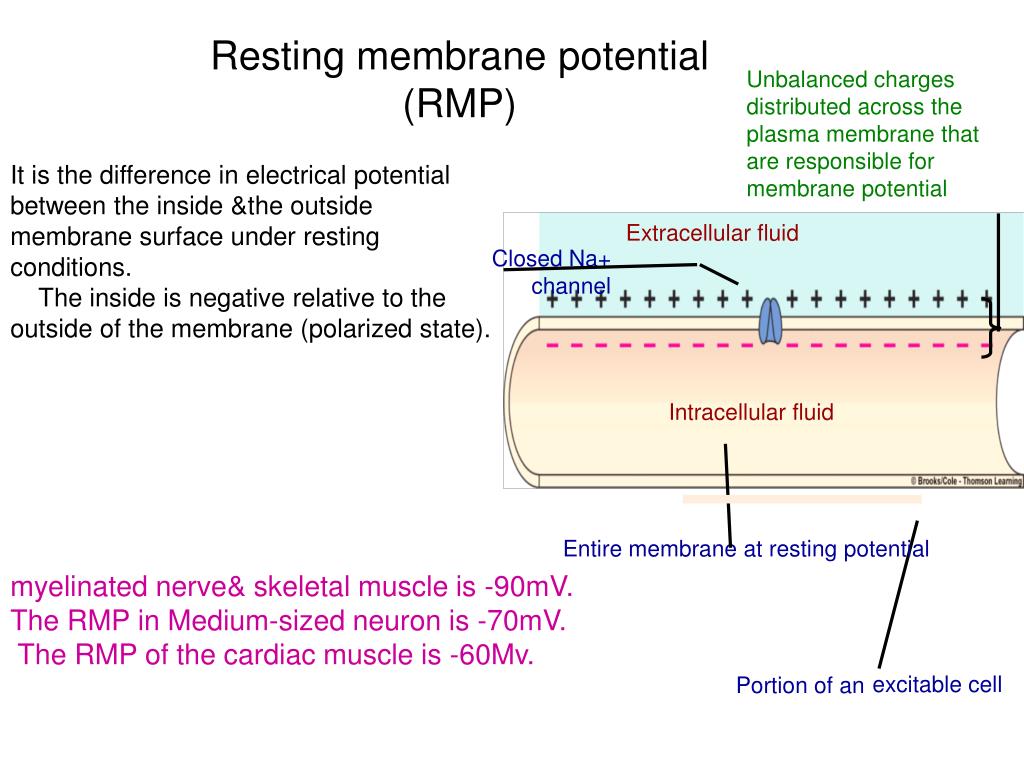
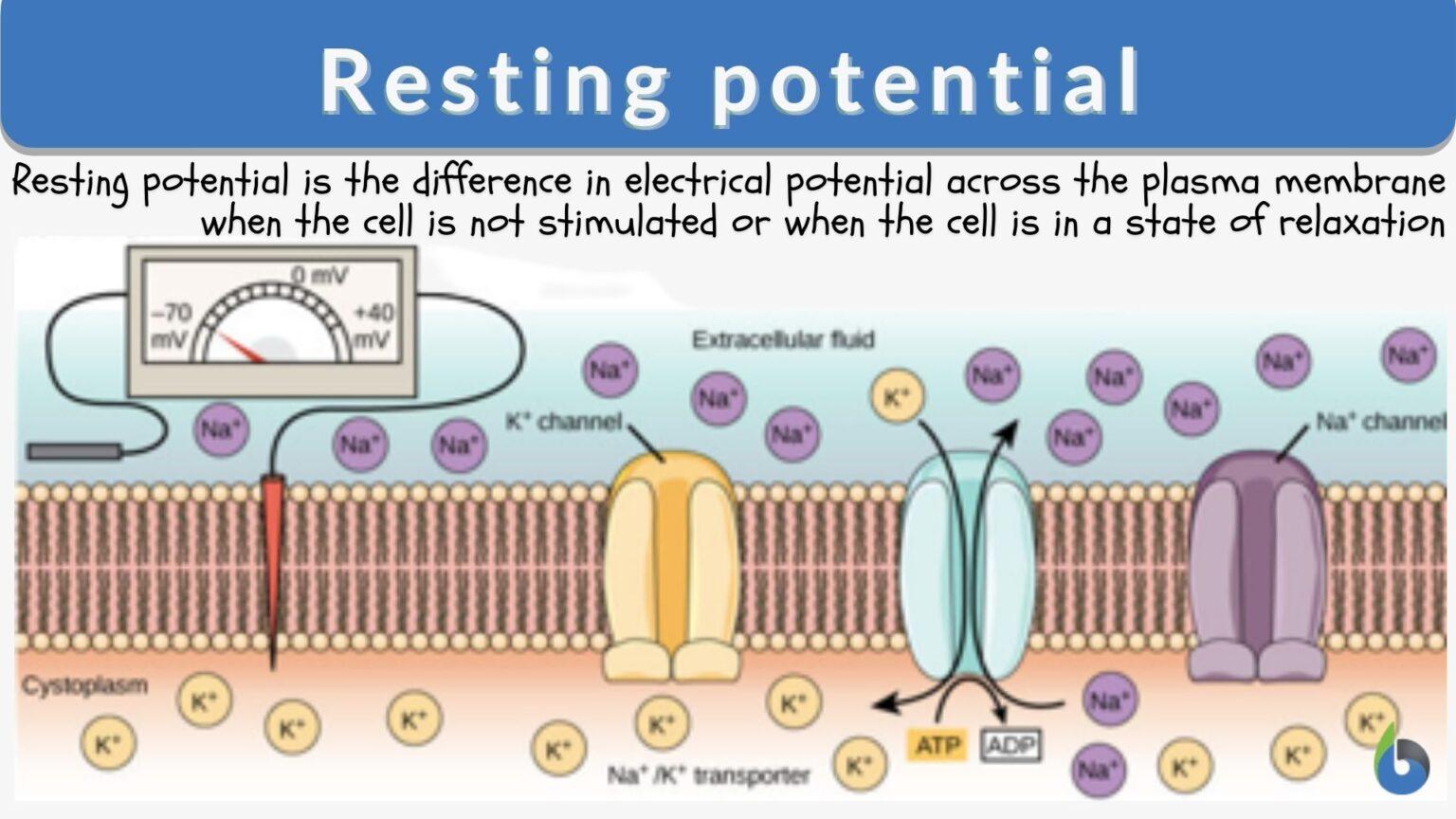
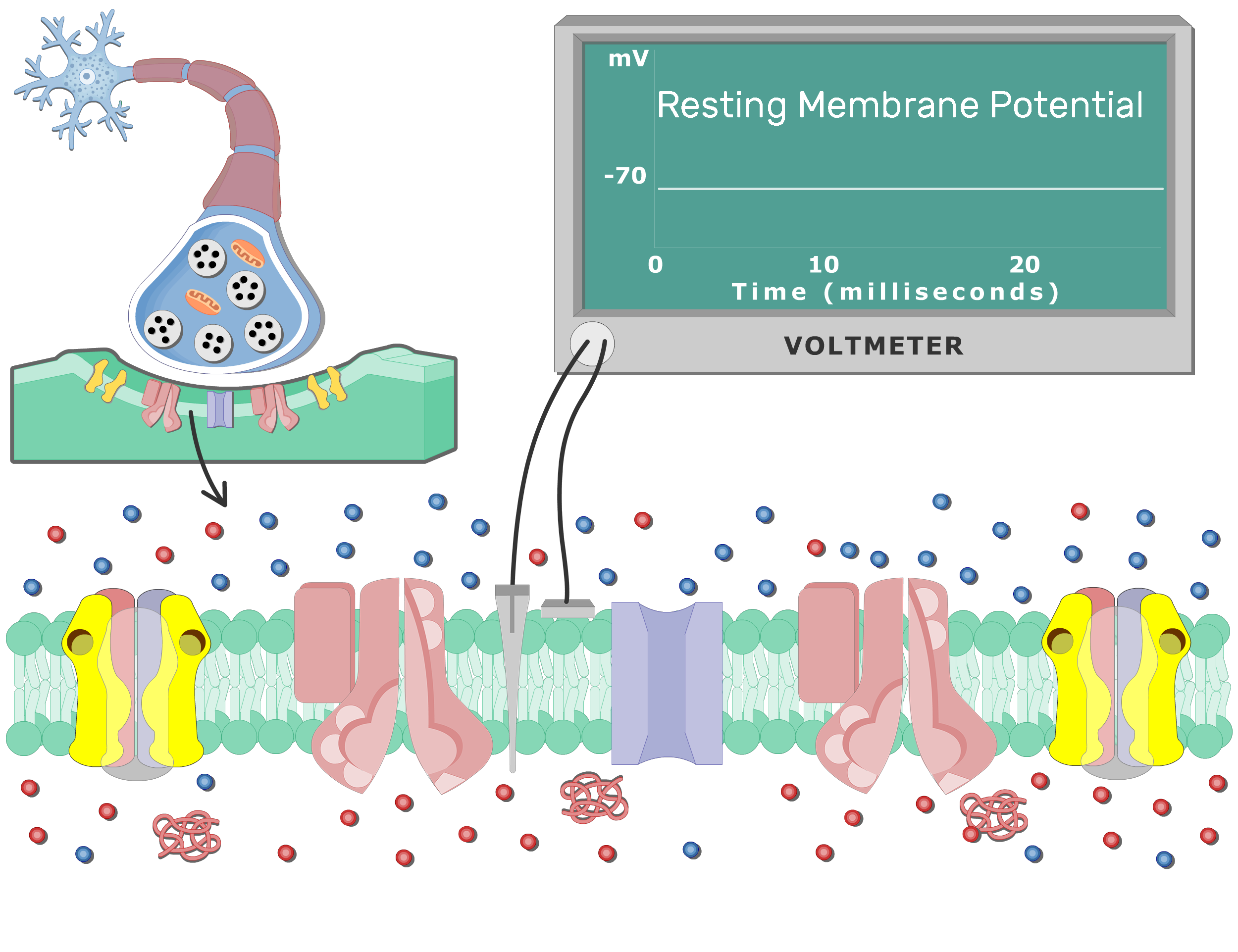
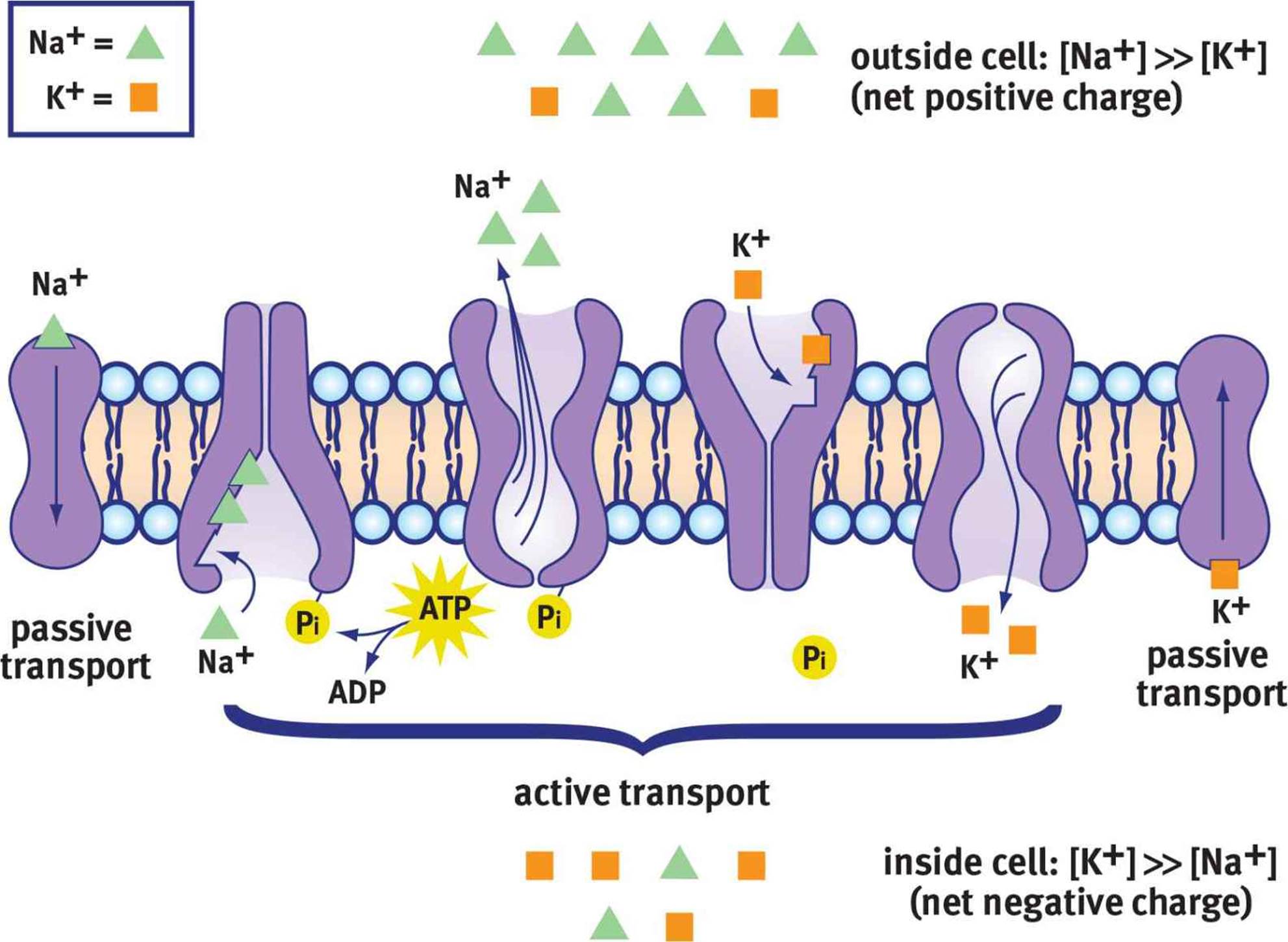
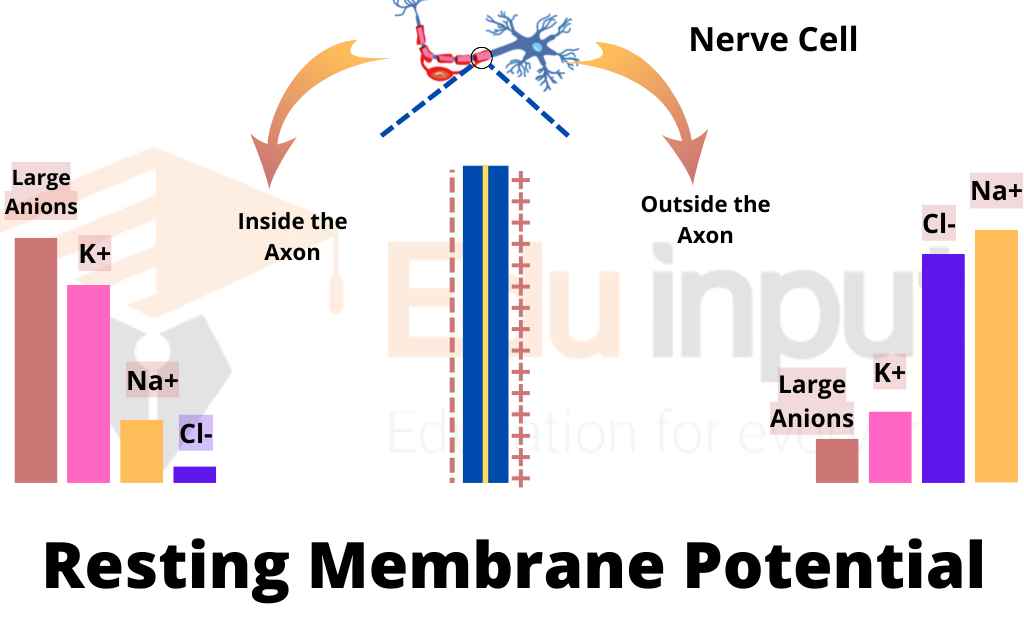
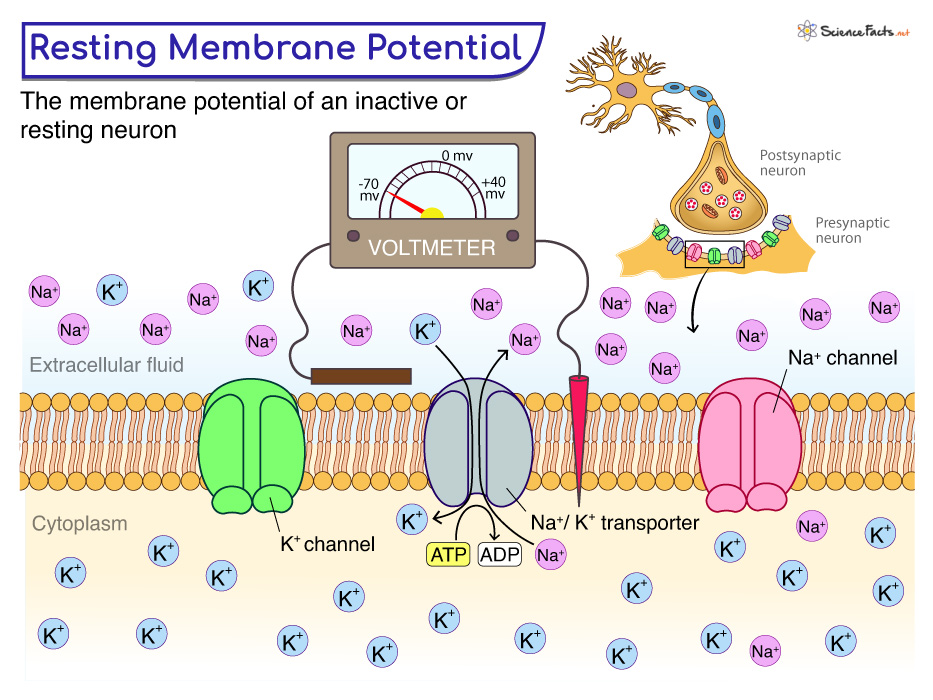
At its core, the resting membrane potential (RMP) is the voltage difference across the cell membrane when the muscle cell is at rest. This difference arises from the unequal distribution of ions, primarily sodium (Na+) and potassium (K+), between the inside and outside of the cell. This distribution is maintained by various factors, including the selective permeability of the cell membrane and the action of the sodium-potassium pump.
The sodium-potassium pump is a crucial protein that actively transports Na+ out of the cell and K+ into the cell, using energy in the form of ATP. This active transport helps to maintain the concentration gradients that drive the resting membrane potential.
In skeletal muscle cells, the RMP is typically around -90 millivolts (mV). This means the inside of the cell is negatively charged compared to the outside.
The Players: Ions and Channels
The key players in establishing and maintaining the RMP are ions – charged particles – and the channels that allow them to cross the cell membrane. These channels can be either leak channels, which are always open, or gated channels, which open or close in response to specific stimuli.
Potassium (K+) ions are more concentrated inside the muscle cell. Leak channels allow K+ to diffuse out of the cell down its concentration gradient. This outward movement of positive charge contributes significantly to the negative resting membrane potential.
Sodium (Na+) ions, on the other hand, are more concentrated outside the cell. While the membrane is less permeable to Na+ at rest, a small amount of Na+ leaks into the cell. The sodium-potassium pump works to counteract this influx.
How It Works: Establishing the Potential
The process of establishing the RMP is a dynamic equilibrium. The outward movement of K+ creates a negative charge inside the cell.
This negative charge attracts K+ back into the cell due to electrical forces. Eventually, an equilibrium is reached where the electrical force pulling K+ in balances the concentration gradient pushing K+ out.
The Nernst equation can be used to calculate the equilibrium potential for each ion, based on its concentration gradient. The Goldman-Hodgkin-Katz equation then integrates these individual potentials, taking into account the relative permeability of the membrane to each ion, to determine the overall resting membrane potential.
Significance for Muscle Contraction
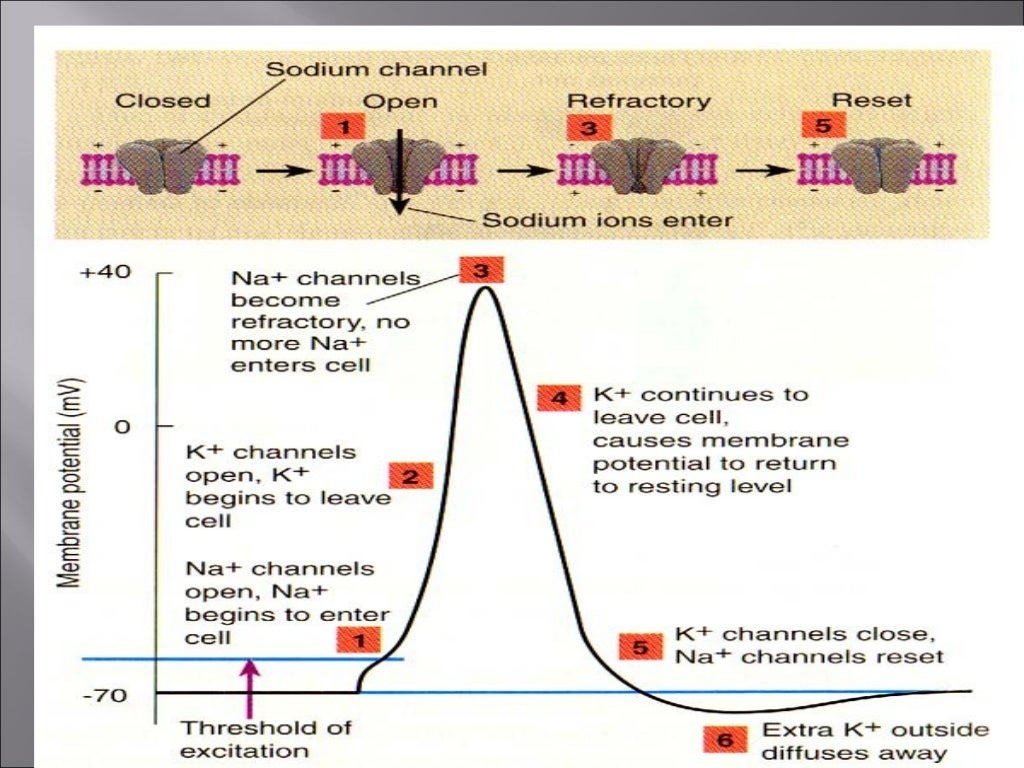
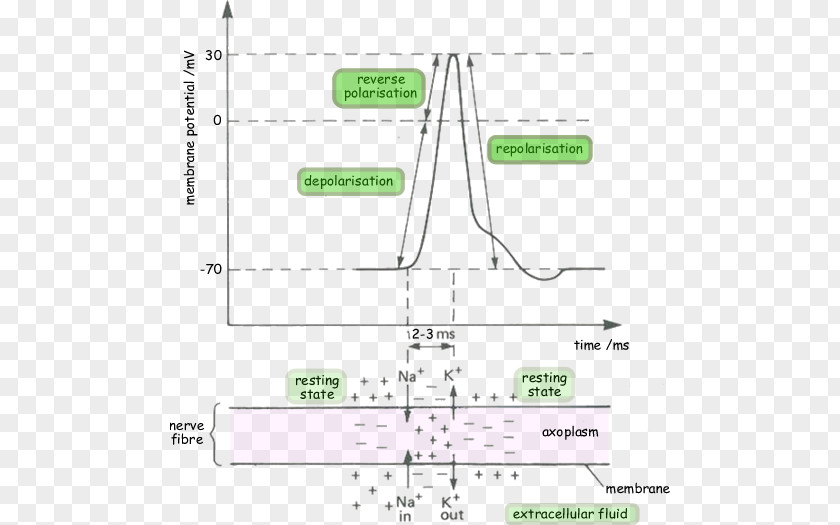
The resting membrane potential is not just a static value; it's a primed state, ready to be disrupted. When a motor neuron stimulates a muscle fiber, it releases a neurotransmitter called acetylcholine.
Acetylcholine binds to receptors on the muscle cell membrane, opening ligand-gated ion channels. This influx of Na+ depolarizes the membrane, making the inside of the cell less negative.
If the depolarization reaches a threshold, it triggers an action potential, a rapid and transient change in membrane potential. This action potential propagates along the muscle fiber, ultimately leading to the release of calcium ions from the sarcoplasmic reticulum and muscle contraction.
Clinical Relevance and Neuromuscular Disorders
Disruptions in the resting membrane potential can have serious consequences. Conditions like hyperkalemia (high potassium levels in the blood) or hypokalemia (low potassium levels) can alter the RMP, making muscle cells more or less excitable.
These changes can lead to muscle weakness, cramps, or even paralysis. Certain genetic disorders, such as some forms of periodic paralysis, directly affect ion channels involved in maintaining the RMP.
Myotonia, a condition characterized by delayed muscle relaxation after voluntary contraction, can also be linked to abnormalities in ion channel function and the resting membrane potential. Researchers are actively investigating these mechanisms to develop targeted therapies.
Future Directions and Research
Ongoing research continues to explore the complexities of the resting membrane potential in skeletal muscle. Scientists are investigating the role of different ion channel subtypes, the impact of various signaling pathways, and the effects of exercise and aging on the RMP.
Advances in techniques like patch-clamp electrophysiology allow researchers to directly measure and manipulate ion channel activity and membrane potential. This provides valuable insights into the fundamental mechanisms of muscle function.
Understanding the intricacies of the resting membrane potential opens doors for developing new strategies to treat neuromuscular disorders, enhance athletic performance, and improve overall muscle health. Targeting specific ion channels involved in RMP regulation represents a promising avenue for therapeutic intervention.