Retinitis Pigmentosamarketed And Pipeline Drugs Analysis

The relentless darkness of Retinitis Pigmentosa (RP), a group of inherited retinal diseases, continues to cast a long shadow over the lives of millions worldwide. While a cure remains elusive, the field is witnessing a surge of innovation, with marketed therapies offering slivers of hope and a robust pipeline promising even brighter prospects for vision preservation and restoration.
This article delves into the current landscape of RP treatments, scrutinizing the efficacy and accessibility of existing options. It will analyze the most promising drugs in the pipeline, examining their mechanisms of action, clinical trial results, and potential to reshape the future for individuals living with this debilitating condition.
Marketed Therapies: A Beacon of Hope in the Darkness
Currently, the approved treatments for RP primarily focus on slowing disease progression and managing associated complications.
Luxturna: Gene Therapy's Pioneering Role
Luxturna (voretigene neparvovec-rzyl), developed by Novartis, stands as a groundbreaking achievement: the first FDA-approved gene therapy for an inherited disease and specifically targeting RP caused by mutations in the RPE65 gene.
This one-time subretinal injection delivers a functional copy of the RPE65 gene to retinal cells, enabling them to produce the necessary protein for light detection.
Clinical trials demonstrated significant improvements in visual function, particularly in children, although long-term durability and accessibility remain key considerations.
Other Marketed Therapies and Management Strategies
Beyond gene therapy, other approaches aim to mitigate the effects of RP and manage its symptoms.
Vitamin A palmitate supplementation has been shown to modestly slow the rate of retinal degeneration in some individuals, though high doses can be toxic.
Protecting the eyes from excessive sunlight with sunglasses and addressing associated conditions like cataracts and macular edema are crucial aspects of comprehensive RP management.
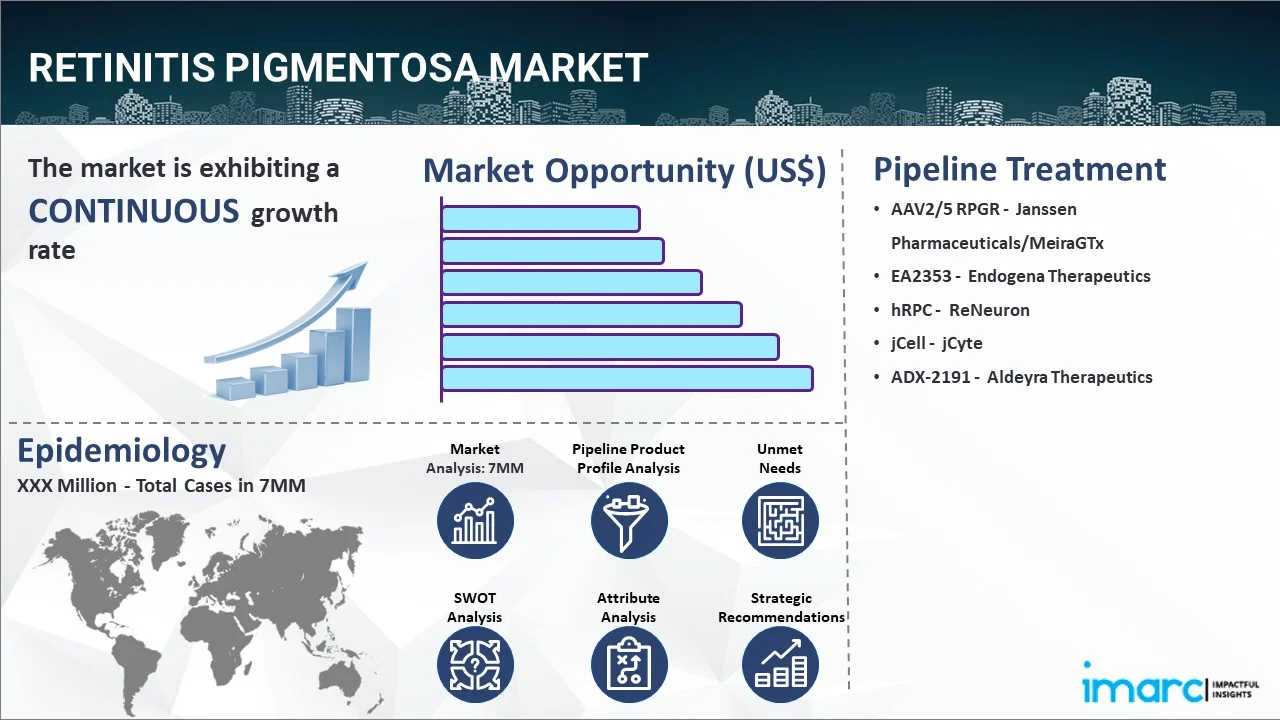
Pipeline Drugs: A Glimmer of Brighter Vision
The RP treatment pipeline is burgeoning with novel therapies targeting various aspects of the disease, from genetic mutations to neuroprotection.
Gene Therapies: Expanding the Reach
Building on the success of Luxturna, researchers are developing gene therapies for other common RP-causing genes.
AGTC-501, under development by Applied Genetic Technologies Corporation (AGTC), targets RP caused by mutations in the RPGR gene, a frequent culprit in X-linked RP.
Early phase trials have shown promising results in terms of safety and potential for visual improvement.
Similarly, other companies are exploring gene therapy approaches for mutations in genes like USH2A and MERTK, aiming to expand the applicability of this transformative treatment modality.
Small Molecule Therapies: Precision Targeting
Small molecule drugs offer another avenue for therapeutic intervention, targeting specific cellular pathways involved in retinal degeneration.
QR-421a, an investigational RNA therapy by ProQR Therapeutics, is designed to address RP caused by a specific mutation (exon 13 skipping) in the USH2A gene, a common cause of RP.
Clinical trial data has shown potential for slowing disease progression and improving visual function in some patients.
Cell-Based Therapies: Rebuilding the Retina
Cell-based therapies hold the potential to replace damaged retinal cells and restore visual function.
Researchers are exploring the use of retinal progenitor cells (RPCs) and induced pluripotent stem cell (iPSC)-derived retinal cells to regenerate photoreceptors and other retinal components.
While still in early stages of development, these approaches offer a tantalizing prospect for reversing vision loss in RP.
Neuroprotective Strategies: Shielding the Retina
Neuroprotective agents aim to protect retinal cells from further damage and slow the rate of degeneration.
Several compounds with neuroprotective properties, such as ciliary neurotrophic factor (CNTF) and brimonidine, are being investigated for their potential to preserve vision in RP.
These strategies focus on bolstering the resilience of remaining retinal cells and extending their lifespan.
Challenges and Future Directions
Despite the progress in RP research and treatment, significant challenges remain.
The genetic heterogeneity of RP, with mutations in over 100 different genes, poses a major hurdle to developing broadly applicable therapies.
Accurate and timely genetic diagnosis is crucial for identifying patients who may benefit from specific gene therapies or targeted treatments.
Furthermore, the cost and accessibility of advanced therapies like gene therapy remain significant barriers for many patients.
"Increased funding for research, streamlined regulatory pathways, and innovative pricing models are needed to ensure that these life-changing treatments reach those who need them most," emphasizes Dr. Emily Carter, a leading RP researcher at the National Eye Institute.
Looking ahead, the future of RP treatment is likely to involve a combination of approaches tailored to individual patients based on their specific genetic mutations and disease characteristics.
Personalized medicine, with therapies designed to address the unique needs of each patient, holds the greatest promise for halting the progression of RP and restoring vision.
Continued innovation in gene therapy, small molecule drugs, cell-based therapies, and neuroprotective strategies will pave the way for a brighter future for individuals living with this devastating condition.



.jpg)