Shoulder Replacement Devices Pipeline Products Market

The shoulder replacement market is bracing for disruption with a surge of innovative devices poised to reshape treatment paradigms. Surgeons and patients alike await advancements promising improved function, longevity, and personalized care.
Fueled by an aging population and rising rates of shoulder osteoarthritis, the pipeline for shoulder replacement devices is brimming with innovation. This wave of new technologies aims to address the limitations of existing implants and procedures, offering hope for better patient outcomes and reduced revision surgeries.
Emerging Technologies in the Pipeline
The landscape of shoulder replacement is evolving rapidly, with several key areas of innovation driving the development of new devices.
Personalized Implants
Companies are investing heavily in patient-specific implants. These custom-designed implants, often created using 3D printing technology, offer a more precise fit and potentially improved biomechanics compared to standard, off-the-shelf devices. The goal is to enhance implant stability and reduce the risk of complications.
Reverse Shoulder Arthroplasty Advancements
Reverse shoulder arthroplasty, particularly for patients with rotator cuff tears, continues to be a focus of innovation. New designs aim to improve range of motion, reduce scapular notching (a common complication), and enhance fixation to the bone. DePuy Synthes and Zimmer Biomet are key players in this segment.
Stemless Implants
Stemless implants are gaining traction, offering a bone-sparing approach to shoulder replacement. These implants require less bone resection and are potentially easier to revise in the future. Several companies, including Exactech, are actively developing and marketing stemless options.
Biologic Coatings and Materials
Researchers are exploring the use of biologic coatings to enhance bone ingrowth and reduce the risk of infection. These coatings may incorporate growth factors or antimicrobial agents to promote faster healing and improve implant longevity. Biocompatible materials are also being investigated to minimize adverse reactions.
Robotic-Assisted Surgery
Robotic-assisted surgery is emerging as a valuable tool for improving the precision and accuracy of shoulder replacement procedures. These systems offer enhanced visualization and control, potentially leading to better implant positioning and reduced soft tissue damage. Stryker is actively developing and deploying robotic systems for shoulder arthroplasty.
Market Dynamics and Key Players
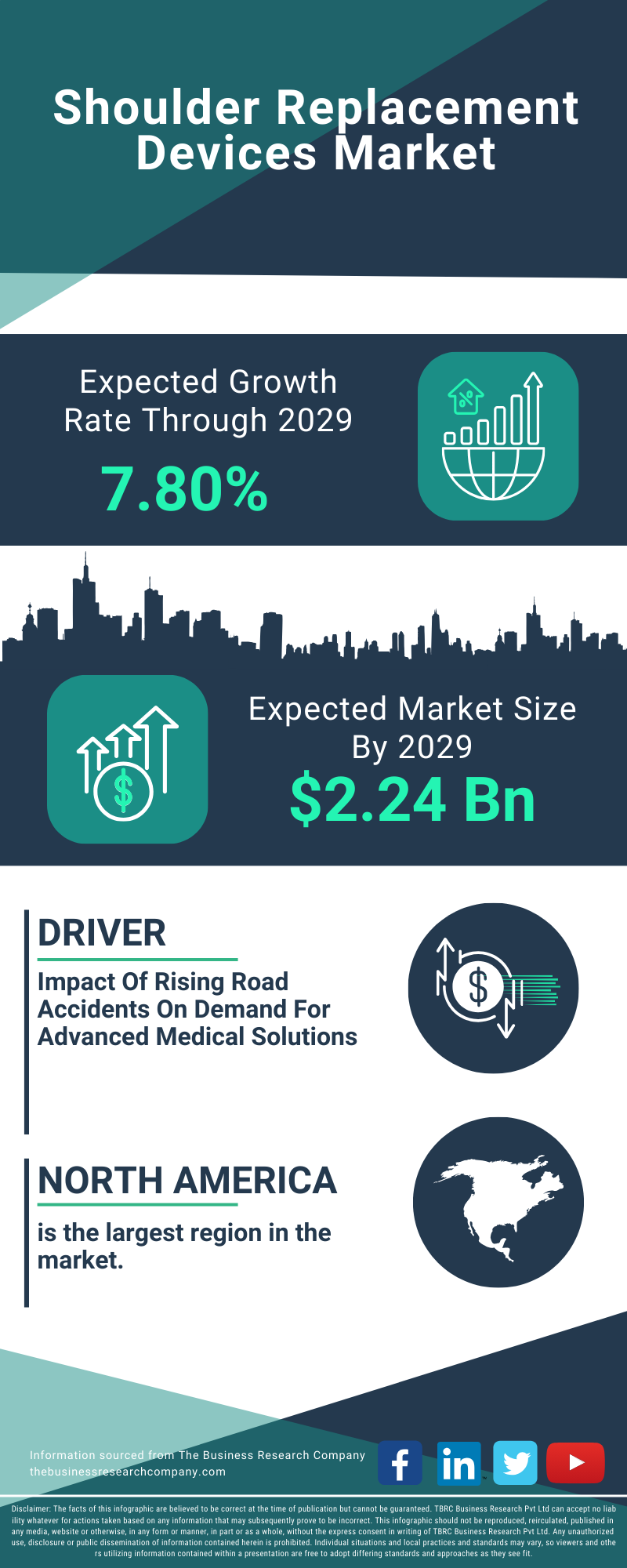
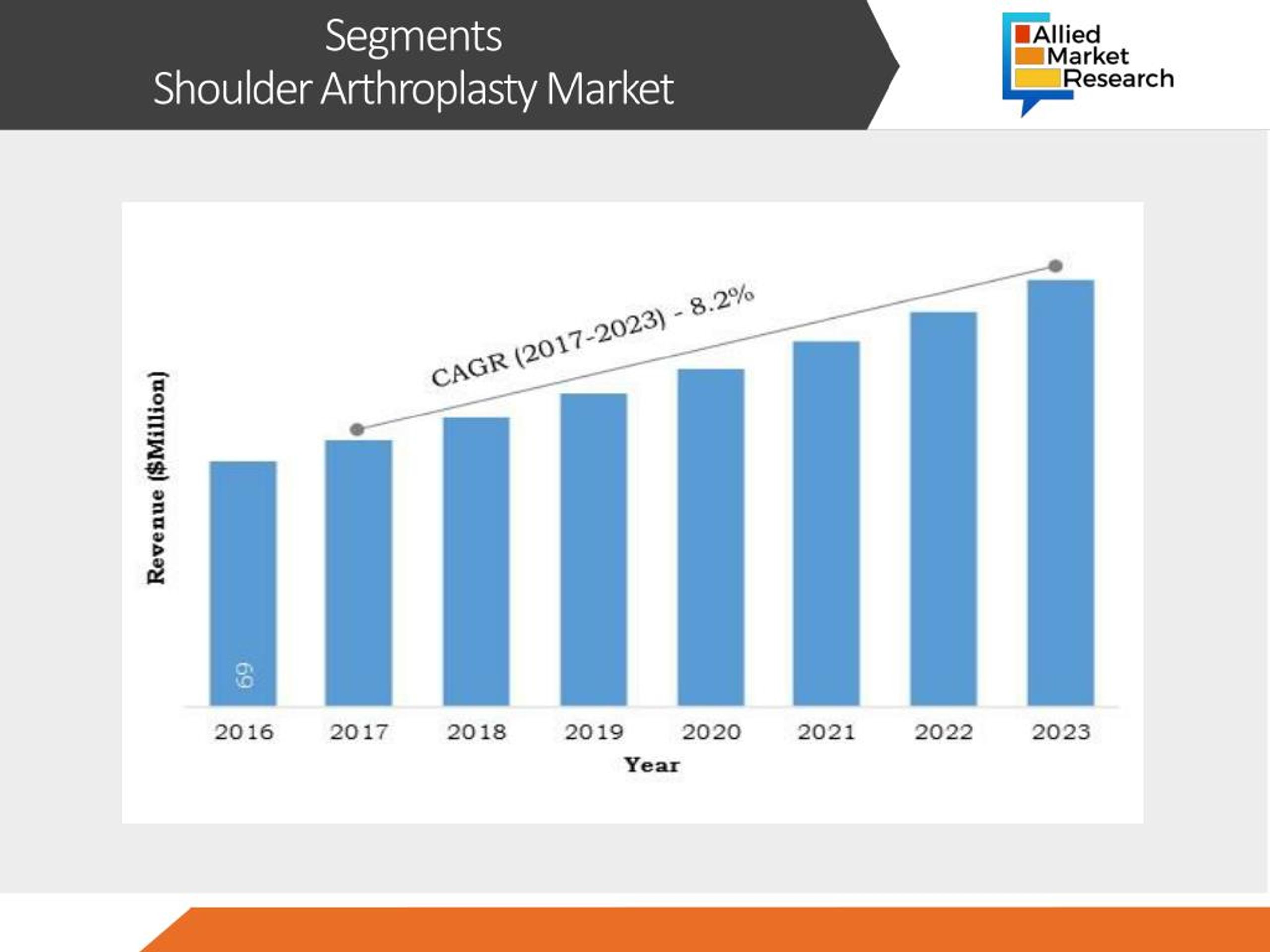
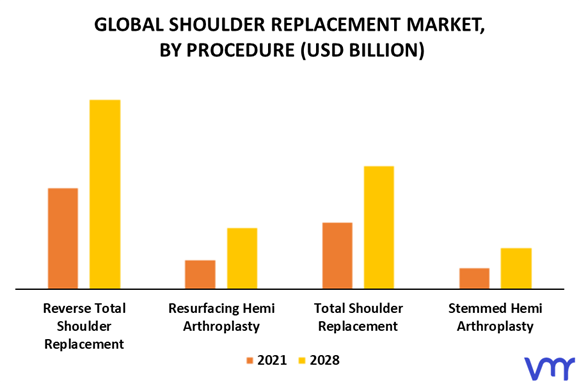
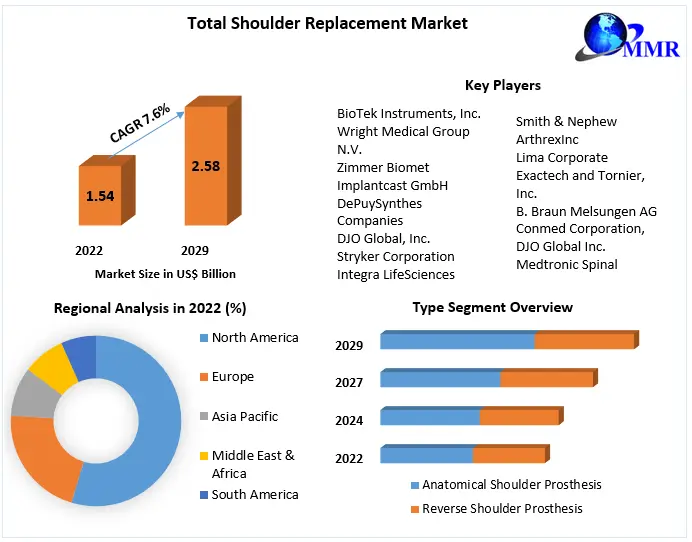
The global shoulder replacement market is a multi-billion dollar industry, with significant growth expected in the coming years.
The major players in the market include: DePuy Synthes (Johnson & Johnson), Zimmer Biomet, Stryker, Smith & Nephew, and Exactech. These companies are investing heavily in research and development to bring innovative products to market.
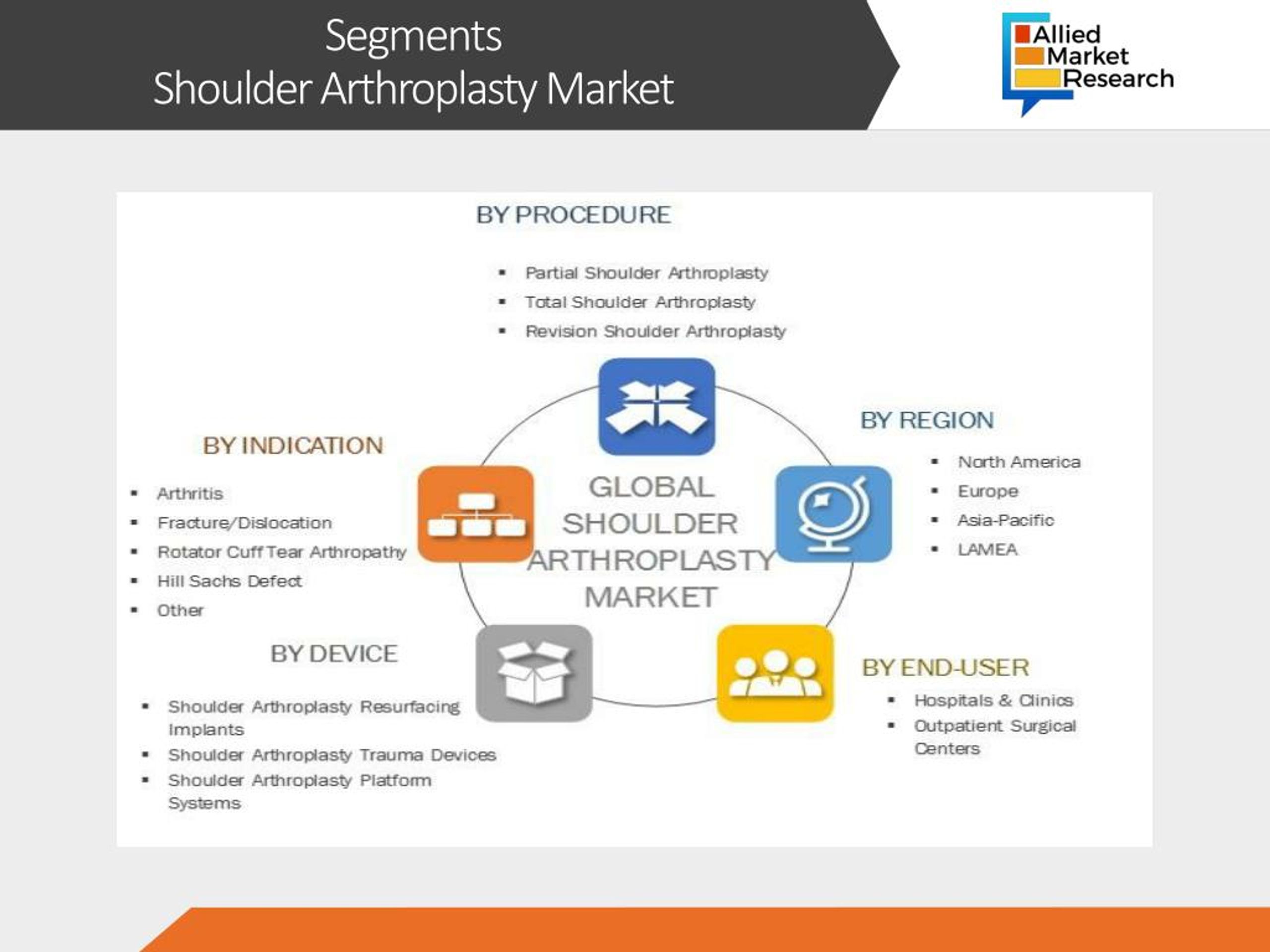
The North American market currently holds the largest share, driven by a large aging population and high healthcare spending. However, the Asia-Pacific region is expected to experience the fastest growth rate, fueled by increasing awareness of shoulder replacement and rising disposable incomes. Market trends indicate a growing demand for minimally invasive procedures and personalized implants globally.
Regulatory Landscape and Approval Pathways
The approval process for shoulder replacement devices varies depending on the regulatory agency. In the United States, the FDA requires manufacturers to demonstrate the safety and effectiveness of their devices through clinical trials.
The 510(k) pathway is commonly used for devices that are substantially equivalent to existing products on the market. However, more innovative devices may require premarket approval (PMA), which involves a more rigorous review process.
In Europe, shoulder replacement devices must meet the requirements of the Medical Device Regulation (MDR). This regulation places a greater emphasis on clinical evidence and post-market surveillance.
Challenges and Opportunities
Despite the promising advancements in the pipeline, several challenges remain. These include the high cost of new technologies, the need for long-term clinical data, and the potential for complications such as infection and implant loosening.
However, the opportunities are significant. The development of more durable, functional, and personalized implants could dramatically improve the quality of life for patients with shoulder pain and disability. Furthermore, the adoption of robotic-assisted surgery and biologic coatings could lead to better outcomes and reduced revision rates.
Looking Ahead
The shoulder replacement market is poised for significant transformation in the coming years. Ongoing clinical trials and regulatory reviews will determine the fate of many promising devices currently in development.
Surgeons and patients should stay informed about the latest advancements in shoulder replacement technology. Continued research and innovation are essential to address the unmet needs of patients with shoulder disorders. The future of shoulder replacement lies in personalized care, enhanced functionality, and improved long-term outcomes.