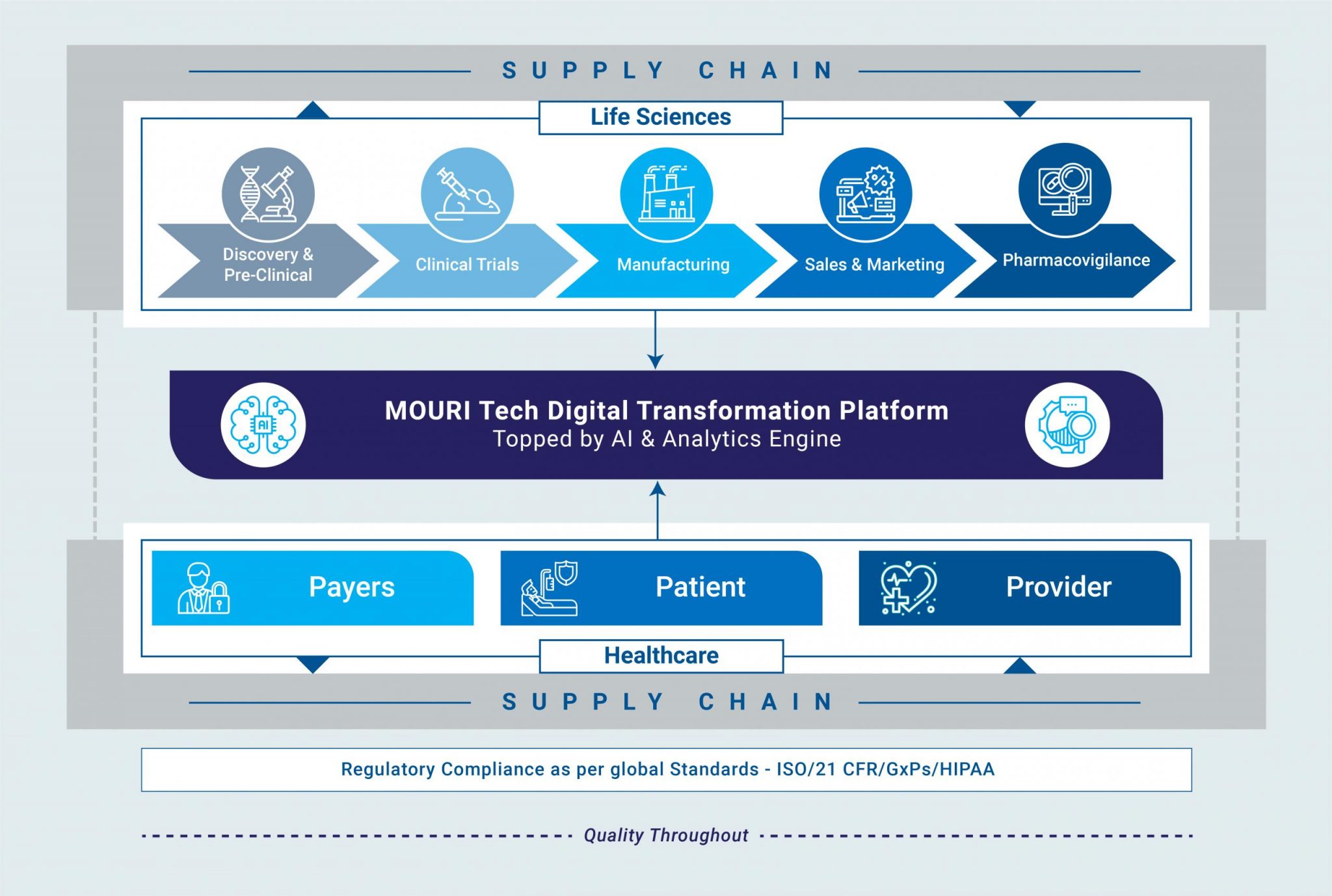
Technical Documentation In Life Sciences Industry

Imagine a bustling laboratory, researchers in crisp white coats hunched over microscopes, the air thick with the scent of discovery. But beyond the whirring centrifuges and complex experiments lies a critical, often unseen force driving innovation in the life sciences: technical documentation. This isn't just about dry manuals; it's the lifeblood of progress, ensuring accuracy, compliance, and ultimately, patient safety.
At its core, technical documentation in the life sciences encompasses all written materials that describe, instruct, and validate the development, manufacturing, and use of medical devices, pharmaceuticals, and other related products. From intricate engineering schematics to detailed clinical trial reports, these documents serve as a vital bridge between groundbreaking research and real-world applications, playing an increasingly pivotal role in an industry governed by stringent regulations and an unwavering commitment to ethical standards.
The Foundation of Compliance and Innovation
The importance of technical documentation in the life sciences stems from its critical role in ensuring regulatory compliance. Organizations like the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe mandate comprehensive documentation throughout the entire product lifecycle.
This documentation serves as evidence that a product is safe, effective, and manufactured to the highest quality standards. Without meticulous records, companies risk facing significant delays in product approvals, costly recalls, and even legal repercussions.
Beyond compliance, well-crafted technical documentation fuels innovation. By meticulously recording every step of the research and development process, companies can build a robust knowledge base.
This enables researchers to learn from past successes and failures, accelerate the development of new therapies, and ultimately bring life-saving treatments to patients more efficiently. It's about capturing not just the "what," but also the "why" behind every decision, creating a living record of scientific progress.
A Multifaceted Landscape
Technical documentation in the life sciences is not a monolithic entity; it encompasses a wide range of documents, each serving a unique purpose. These include:
Design Documentation
These documents detail the design specifications, engineering drawings, and testing protocols for medical devices and pharmaceutical products. They provide a comprehensive overview of how a product is intended to function and how its performance will be evaluated.
Manufacturing Documentation
This category includes standard operating procedures (SOPs), batch records, and quality control reports. These documents ensure that products are manufactured consistently and meet stringent quality standards.
Clinical Trial Documentation
Clinical trial reports, investigator brochures, and patient consent forms fall under this category. These documents are essential for demonstrating the safety and efficacy of new treatments before they can be approved for use.
Regulatory Submissions
These documents are submitted to regulatory agencies like the FDA and EMA to obtain approval for new products. They include a comprehensive overview of the product's development, manufacturing, and clinical trial data.
User Manuals and Instructions for Use (IFUs)
These documents provide detailed instructions on how to use a medical device or pharmaceutical product safely and effectively. Clear and concise instructions are critical for ensuring patient safety and preventing misuse.
Challenges and Opportunities in the Digital Age
The life sciences industry is undergoing a digital transformation, and technical documentation is no exception. The shift from paper-based systems to electronic document management systems (EDMS) has brought significant benefits, including improved efficiency, enhanced collaboration, and reduced costs.
However, this transition also presents challenges. Ensuring data integrity, maintaining version control, and complying with data privacy regulations are just some of the hurdles that companies must overcome.
Furthermore, the increasing complexity of medical devices and pharmaceutical products demands more sophisticated documentation strategies. Artificial intelligence (AI) and machine learning (ML) are beginning to play a role in automating document creation, improving content quality, and enhancing searchability.
For example, AI-powered tools can be used to generate summaries of complex clinical trial reports or to identify inconsistencies in manufacturing documentation. This not only saves time and resources but also reduces the risk of human error.
The Human Element
While technology plays an increasingly important role, the human element remains crucial in technical documentation. Skilled technical writers are essential for translating complex scientific and technical information into clear, concise, and accessible language.
They must possess a deep understanding of the subject matter, excellent writing skills, and a strong attention to detail. Moreover, they must be able to collaborate effectively with scientists, engineers, and regulatory experts.
The role of the technical writer is evolving from simply documenting information to actively shaping the user experience. By creating user-friendly manuals and intuitive online help systems, technical writers can empower patients and healthcare professionals to use medical devices and pharmaceutical products safely and effectively.
Looking Ahead: The Future of Technical Documentation
The future of technical documentation in the life sciences is likely to be shaped by several key trends. These include:
- Increased Focus on Data Integrity: Regulatory agencies are placing increasing emphasis on data integrity and traceability. Companies will need to implement robust systems and processes to ensure that their documentation is accurate, complete, and reliable.
- Greater Use of Automation: AI and ML will continue to automate document creation, improve content quality, and enhance searchability.
- Personalized Documentation: As medical devices and pharmaceutical products become more personalized, so too will the documentation that supports them.
- Enhanced Collaboration: Cloud-based document management systems will facilitate collaboration between stakeholders across different departments and geographical locations.
Ultimately, the goal of technical documentation in the life sciences is to ensure that medical devices and pharmaceutical products are safe, effective, and accessible to all who need them. By embracing new technologies, investing in skilled technical writers, and maintaining a relentless focus on quality, the industry can continue to improve patient outcomes and drive innovation.
Investing in Excellence
In conclusion, technical documentation is far more than just a regulatory requirement; it is a strategic asset that can drive innovation, improve patient safety, and enhance brand reputation. Companies that invest in high-quality documentation will be well-positioned to succeed in the increasingly competitive and highly regulated life sciences industry.
It's a commitment to clarity, accuracy, and transparency – a testament to the industry's dedication to improving lives through science and technology. The next time you see a seemingly simple instruction manual, remember the intricate web of knowledge, expertise, and diligence that lies beneath its surface, quietly working to ensure the health and well-being of us all.