Term Used For Compounds With Properties For Neutralizing Acids

Imagine a world where the sharp tang of lemon juice loses its sting, where the burn of indigestion fades into gentle relief. It's a world made possible by an unsung hero of chemistry: a class of compounds with the remarkable ability to tame acidity. These substances, present in everything from the antacids in your medicine cabinet to the fertile soil in your garden, are vital to our health and environment.
This brings us to the heart of the matter: the term used for these acid-neutralizing compounds. It's a word you've likely heard, perhaps in a science class or while browsing the pharmacy aisle: bases. These chemical workhorses play a critical role in maintaining balance, both in our bodies and the world around us.
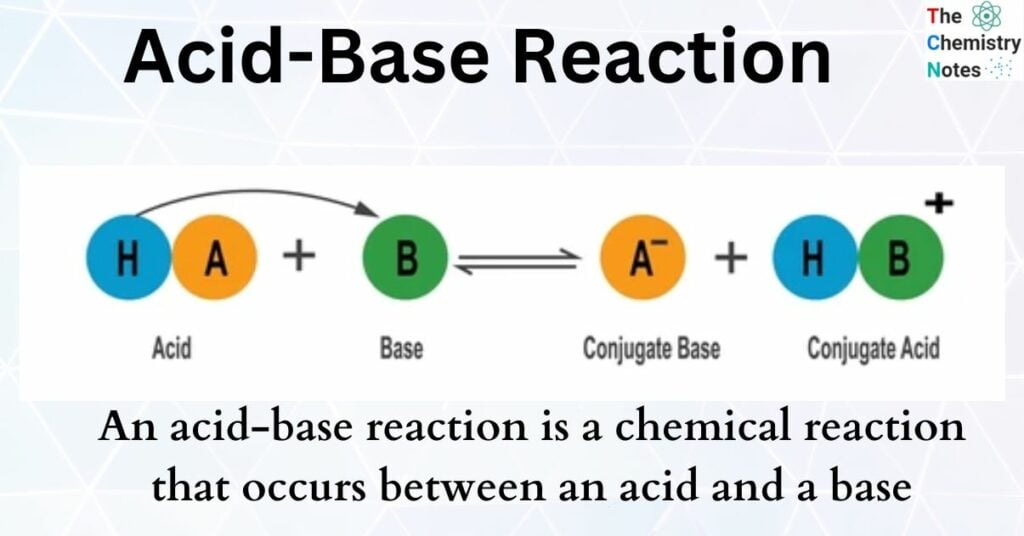
Bases, sometimes called alkalis, are substances that react with acids to neutralize them. This neutralization process forms a salt and water. Understanding bases requires delving into their history, significance, and the science behind their acid-taming power.
A Brief History of Understanding Bases
The concept of bases and acids has evolved over centuries. Early alchemists recognized substances with opposing properties, observing their interactions and effects. However, a deeper understanding had to wait for the development of modern chemistry.
The term "alkali" itself has ancient roots, derived from the Arabic word "al-qali," meaning "the ashes." This refers to the traditional method of obtaining alkaline substances from the ashes of burned plants.
The 17th century saw significant advances. Scientists started to observe the unique properties of substances that could neutralize acids. It was a pivotal moment in the development of the understanding of acids and bases.
In the late 19th century, Svante Arrhenius introduced his theory of electrolytic dissociation. He defined acids as substances that produce hydrogen ions (H+) in water and bases as substances that produce hydroxide ions (OH-) in water.
Arrhenius's theory revolutionized the understanding of acids and bases. It provided a clear and quantifiable way to define and differentiate them. However, his theory was limited to aqueous solutions.
Beyond Arrhenius: Expanding the Definition
The limitations of the Arrhenius theory prompted scientists to seek more comprehensive definitions. Johannes Nicolaus Brønsted and Thomas Martin Lowry independently proposed a broader definition in 1923.
The Brønsted-Lowry theory defines acids as proton (H+) donors and bases as proton acceptors. This definition is not limited to aqueous solutions, extending the concept of acid-base reactions to a wider range of chemical systems.
G.N. Lewis proposed an even more general definition of acids and bases. Lewis acids are electron pair acceptors, and Lewis bases are electron pair donors. This definition encompasses reactions beyond proton transfer, offering the most comprehensive view of acid-base interactions.
The Significance of Bases
Bases are essential in numerous applications, from industrial processes to biological systems. Their ability to neutralize acids makes them vital in many aspects of our lives.
In medicine, antacids containing bases like magnesium hydroxide or calcium carbonate neutralize excess stomach acid. This provides relief from heartburn and indigestion. These are vital in maintaining the balance within the body.
In agriculture, the acidity of soil can be neutralized with bases like lime (calcium oxide). This creates a more favorable environment for plant growth. This also ensures the plant is getting enough nutrients.
Many cleaning products contain bases like ammonia or sodium hydroxide (lye). They are effective at dissolving fats and oils, making them powerful cleaning agents. These should be used with caution, however.
The manufacturing of soaps relies heavily on bases. Saponification is the process of reacting fats or oils with a strong base to create soap. Soap is important for hygiene.
"The importance of bases cannot be overstated. They are essential for a wide range of applications, from maintaining human health to supporting industrial processes." – Dr. Emily Carter, Professor of Chemistry
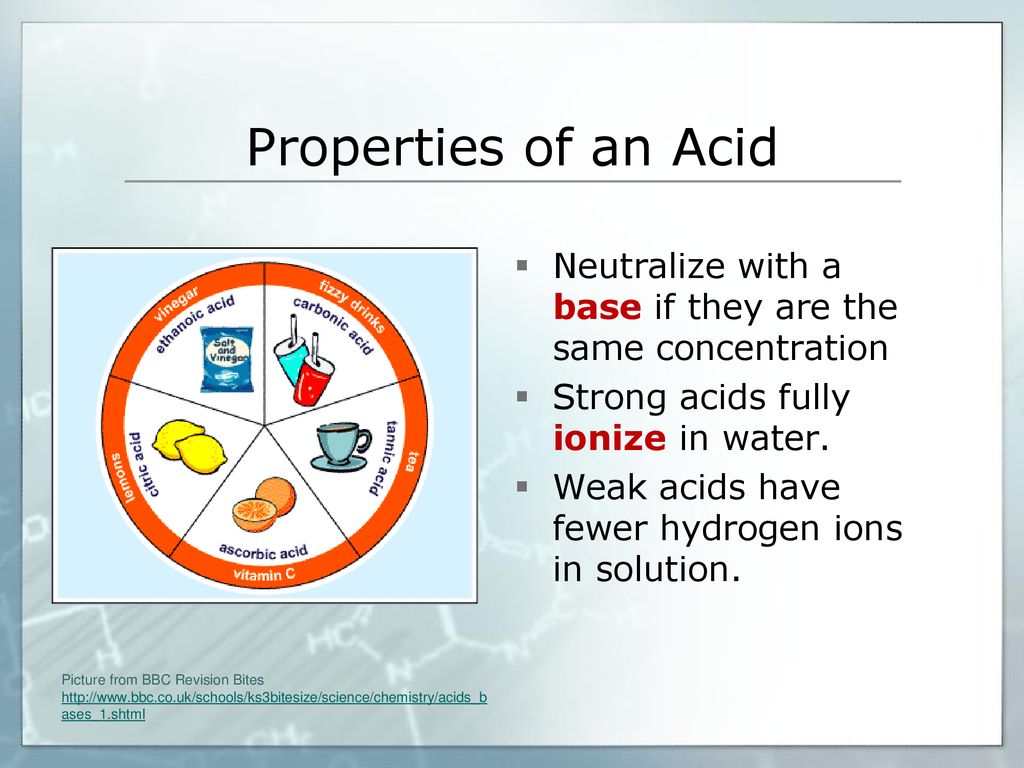
Examples of Common Bases
Bases exist in various forms and strengths. Some are common household items, while others are used primarily in industrial settings.
Sodium hydroxide (NaOH), also known as lye, is a strong base used in soap making, paper production, and drain cleaners. It's a highly corrosive substance and must be handled with care.
Ammonia (NH3) is a weak base used in cleaning products and fertilizers. It is known for its pungent odor.
Calcium hydroxide (Ca(OH)2), also known as slaked lime, is used in agriculture to neutralize acidic soils. It is also used in construction.
Magnesium hydroxide (Mg(OH)2) is a mild base used in antacids and laxatives. It helps neutralize stomach acid and relieve constipation.
Potassium hydroxide (KOH) is a strong base similar to sodium hydroxide. It's used in the production of liquid soaps and detergents.
The pH Scale: Measuring Acidity and Basicity
The pH scale is a logarithmic scale used to measure the acidity or basicity of a solution. It ranges from 0 to 14, with 7 being neutral.
A pH less than 7 indicates acidity, while a pH greater than 7 indicates basicity. The lower the pH, the stronger the acid; the higher the pH, the stronger the base. Understanding the pH scale is crucial for controlling chemical reactions and maintaining optimal conditions in various applications.
For example, a pH of 2 is strongly acidic (like lemon juice), while a pH of 12 is strongly basic (like bleach). Maintaining the right pH is critical in many applications.
Bases in Biological Systems
Bases play a crucial role in biological systems, maintaining the delicate pH balance necessary for life. Enzymes, for example, function optimally within a narrow pH range.
The human body has several buffering systems to maintain a stable pH. Blood, for instance, contains bicarbonate ions, which act as a buffer to neutralize excess acid or base.
Even slight deviations from the normal pH range can have serious consequences for biological processes. This highlights the importance of pH regulation in living organisms.
A Continuing Exploration
Our understanding of bases continues to evolve. Researchers are constantly discovering new applications and refining existing technologies. The study of bases extends to super bases and advanced materials.
The future holds exciting possibilities for the use of bases in novel ways. From developing more efficient energy storage devices to creating innovative materials, the potential of these acid-neutralizing compounds is vast.
Ultimately, the story of bases is a story of balance. It's a reminder that in chemistry, and in life, finding equilibrium is key. Just as a dash of baking soda can temper the tartness of a tomato sauce, bases work to neutralize acidity and create a more harmonious world.






+solution.+Acids+neutralize+bases+in+a+neutralization+reaction..jpg)



.PNG)

.jpg)
+when+dissolved+in+water.+Bases+have+these+properties:.jpg)