The Development Of Antiviral Drug Therapy Is Difficult Because
The global fight against viral diseases, from influenza to HIV and the looming threat of novel pathogens, hinges on the development of effective antiviral drugs. Yet, the path to creating these life-saving medications is fraught with challenges, hindering progress and leaving populations vulnerable.
The development of antiviral drug therapy is difficult because viruses are masters of adaptation and evasion. This article explores the multifaceted obstacles that researchers face in their quest to conquer viral infections, highlighting the scientific complexities, economic disincentives, and regulatory hurdles that impede the development of new antiviral treatments.
The Intricate Nature of Viruses
One of the primary challenges lies in the very nature of viruses. Unlike bacteria, which are complex, self-replicating organisms, viruses are remarkably simple, consisting of genetic material (DNA or RNA) encased in a protein coat.
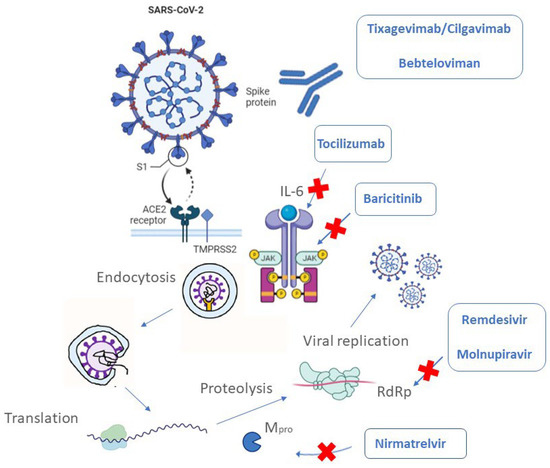
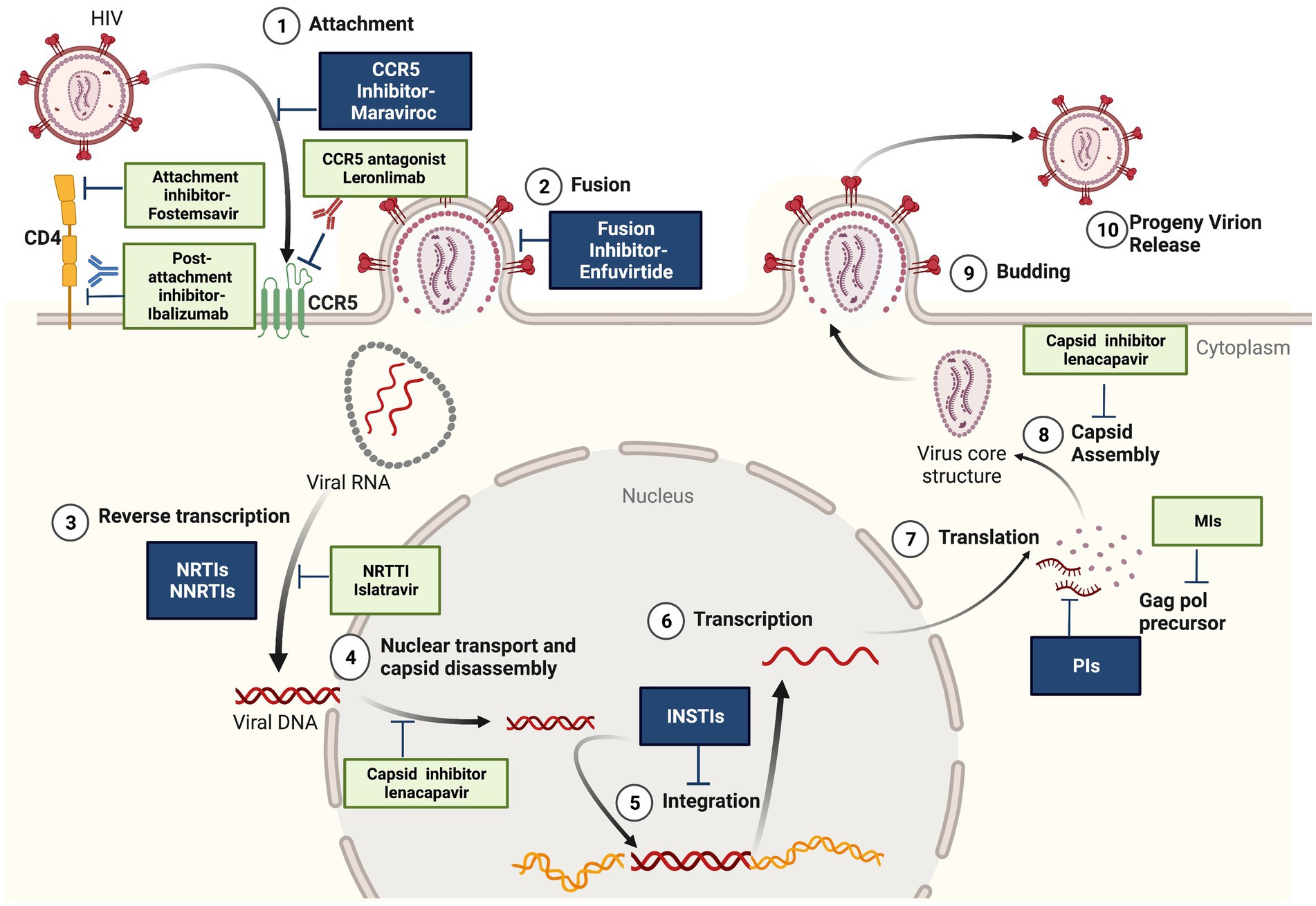
Viruses hijack the host cell's machinery to replicate, essentially reprogramming it to produce more viruses. This intimate relationship with the host cell makes it difficult to target the virus without also harming the host, leading to potential toxicity and side effects.
Dr. Eleanor Vance, a virologist at the National Institutes of Health (NIH), explains, "Developing antivirals is like trying to dismantle a bomb inside a building without damaging the building itself. The closer the virus replicates to the host's normal cellular processes, the harder it becomes to selectively inhibit it."
Viral Mutation and Resistance
Viruses are notorious for their high mutation rates. This rapid evolution allows them to quickly adapt to antiviral drugs, developing resistance mechanisms that render the medications ineffective.
This is particularly problematic for viruses with RNA genomes, such as HIV and influenza, which lack the proofreading mechanisms present in DNA replication. As a result, mutations arise more frequently, accelerating the development of drug resistance.
"We are constantly playing catch-up with viruses," says Dr. Vance. "As soon as we develop a new antiviral, the virus starts mutating and finding ways to circumvent its action. It's a never-ending battle."
The Target Conundrum
Identifying suitable targets for antiviral drugs is another significant hurdle. Ideally, these targets should be unique to the virus and essential for its replication. However, many viral proteins share similarities with host cell proteins, making it difficult to design drugs that selectively inhibit the virus.
Furthermore, some viruses establish latency, hiding within the host cell in a dormant state. During latency, the virus is not actively replicating, making it difficult to target with antiviral drugs.
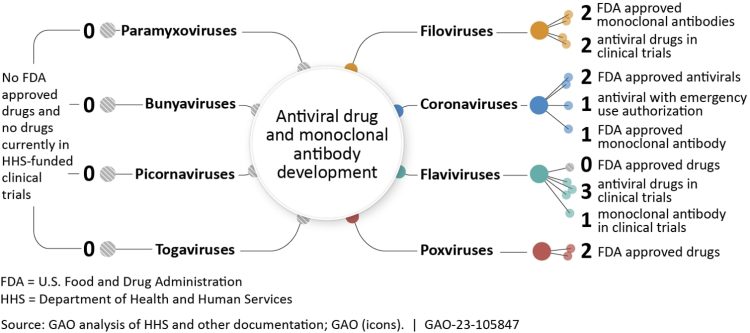
These difficulties increase the development cost. For example, only a small number of drugs is available to treat diseases such as dengue fever.
Economic Disincentives
The high cost and risk associated with antiviral drug development, coupled with the relatively small market for some viral diseases, can create economic disincentives for pharmaceutical companies. Developing new medicines is expensive, and it can be more difficult to develop medicines for those who don't have access to the funding for it.
Many viral infections are self-limiting, meaning they resolve on their own without treatment. This reduces the potential market for antiviral drugs, making it less attractive for companies to invest in their development. Rare viral infections, for example, can be very difficult to get companies to invest in.
Furthermore, the development of broad-spectrum antivirals, which can target multiple viruses, is often challenging. The specificity that comes with medications designed for individual diseases may be lost by medicines designed for multiple.
Regulatory Hurdles
The regulatory approval process for antiviral drugs is rigorous, requiring extensive preclinical and clinical testing to demonstrate safety and efficacy. This process can be lengthy and expensive, adding to the overall cost of drug development.
In addition, the emergence of new viral threats, such as COVID-19, often necessitates an accelerated regulatory pathway. However, this can raise concerns about the thoroughness of the evaluation process and the potential for unforeseen side effects.
Many regulatory bodies, such as the FDA and the EMEA, are also involved with regulating the use of antiviral drugs, often leading to debates over their proper use.
The Path Forward
Despite these challenges, progress is being made in the development of antiviral drugs. New technologies, such as high-throughput screening and structure-based drug design, are accelerating the discovery process.
Furthermore, increased collaboration between academic researchers, pharmaceutical companies, and government agencies is fostering innovation. This increase in collaboration is essential if new antiviral medications are to be developed to fight off future viruses.
The development of effective antiviral drug therapy remains a complex and ongoing challenge. Overcoming the scientific, economic, and regulatory hurdles requires sustained investment in research, innovative approaches, and collaborative efforts. The global health security depends on it.

.jpg)