Through Which Mechanism Is Duchenne Muscular Dystrophy Acquired
Duchenne Muscular Dystrophy (DMD), a devastating genetic disorder primarily affecting males, progressively weakens muscles, leading to significant disability and reduced lifespan. Understanding the precise mechanism through which this disease is acquired is crucial for developing effective treatments and potential cures. This article delves into the genetic basis of DMD, explaining how mutations in a specific gene lead to the disease's characteristic symptoms.
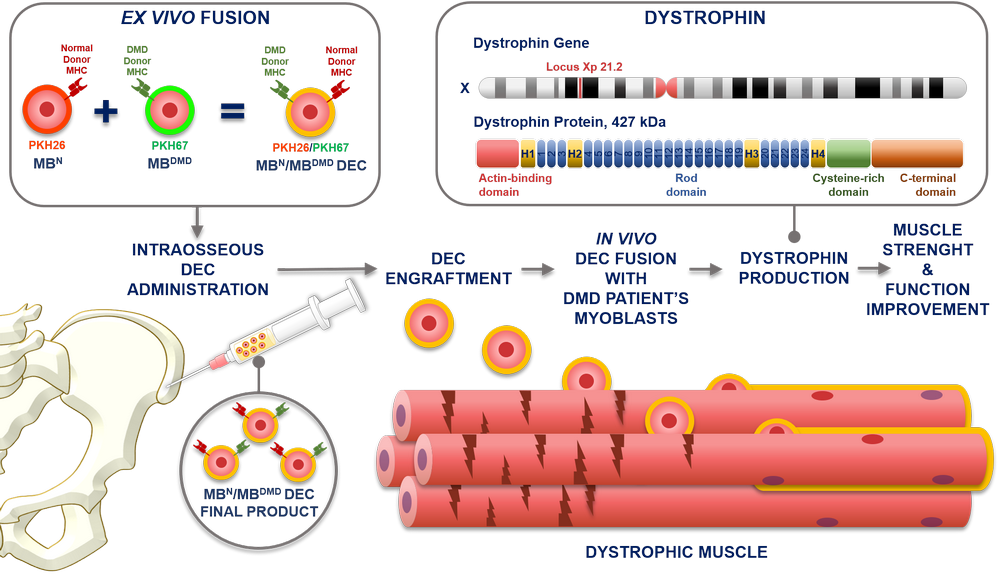
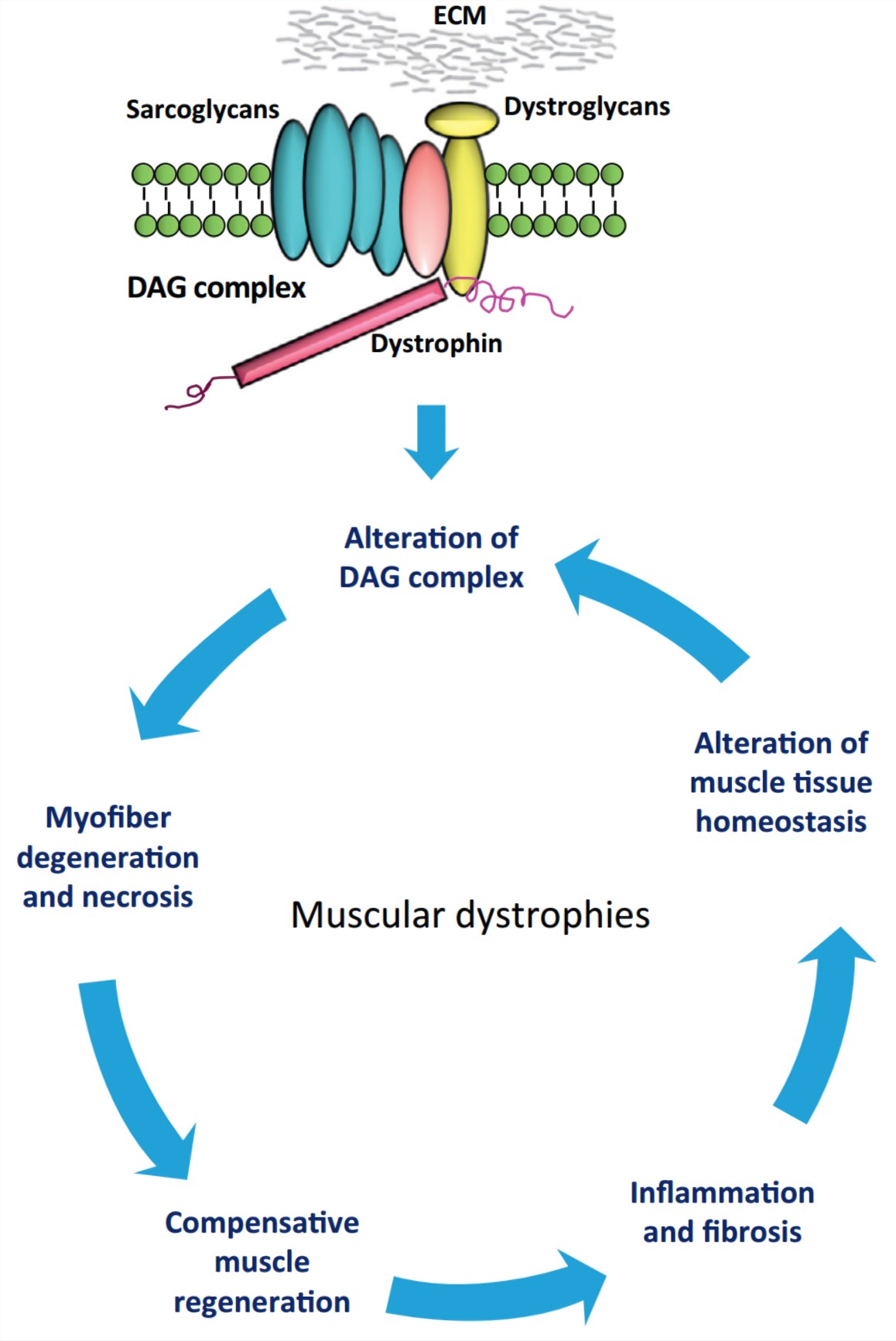
At the heart of DMD lies a faulty gene responsible for producing dystrophin, a protein vital for muscle fiber stability. This gene, located on the X chromosome, is the largest known gene in the human genome, making it particularly susceptible to mutations. Consequently, a range of genetic errors can disrupt dystrophin production, leading to the manifestation of DMD.
The Genetic Defect: A Breakdown
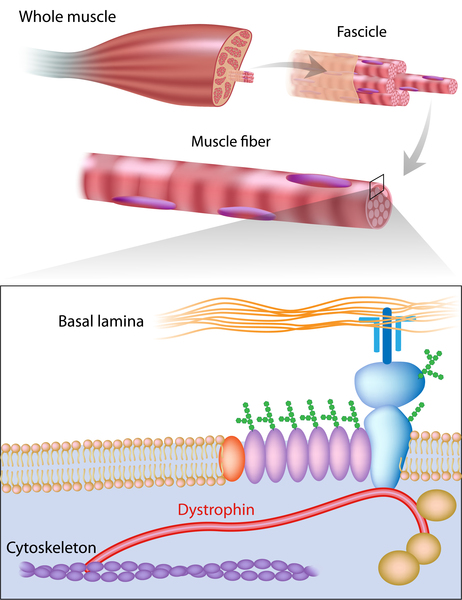
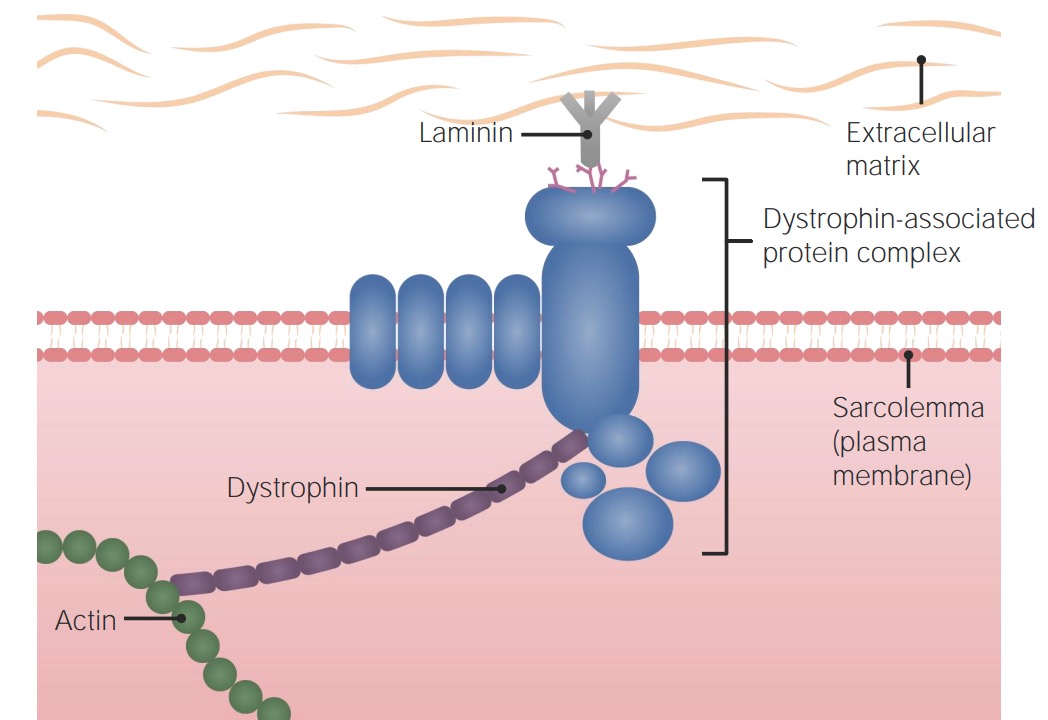
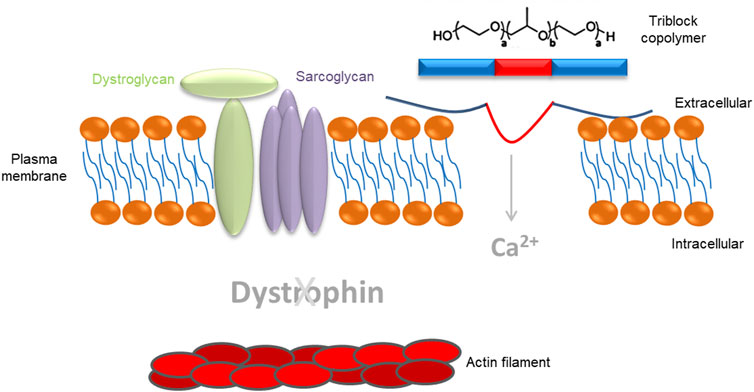
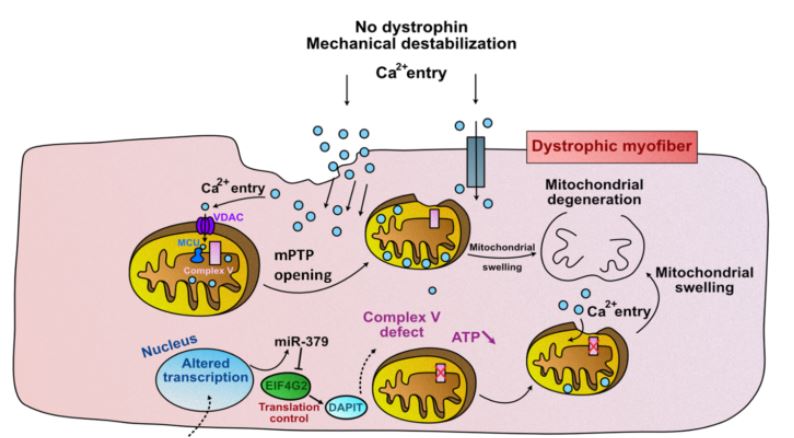
The dystrophin gene provides instructions for making the dystrophin protein. Dystrophin acts like a shock absorber, connecting the muscle fibers' internal scaffolding (cytoskeleton) to the extracellular matrix, the surrounding tissue.
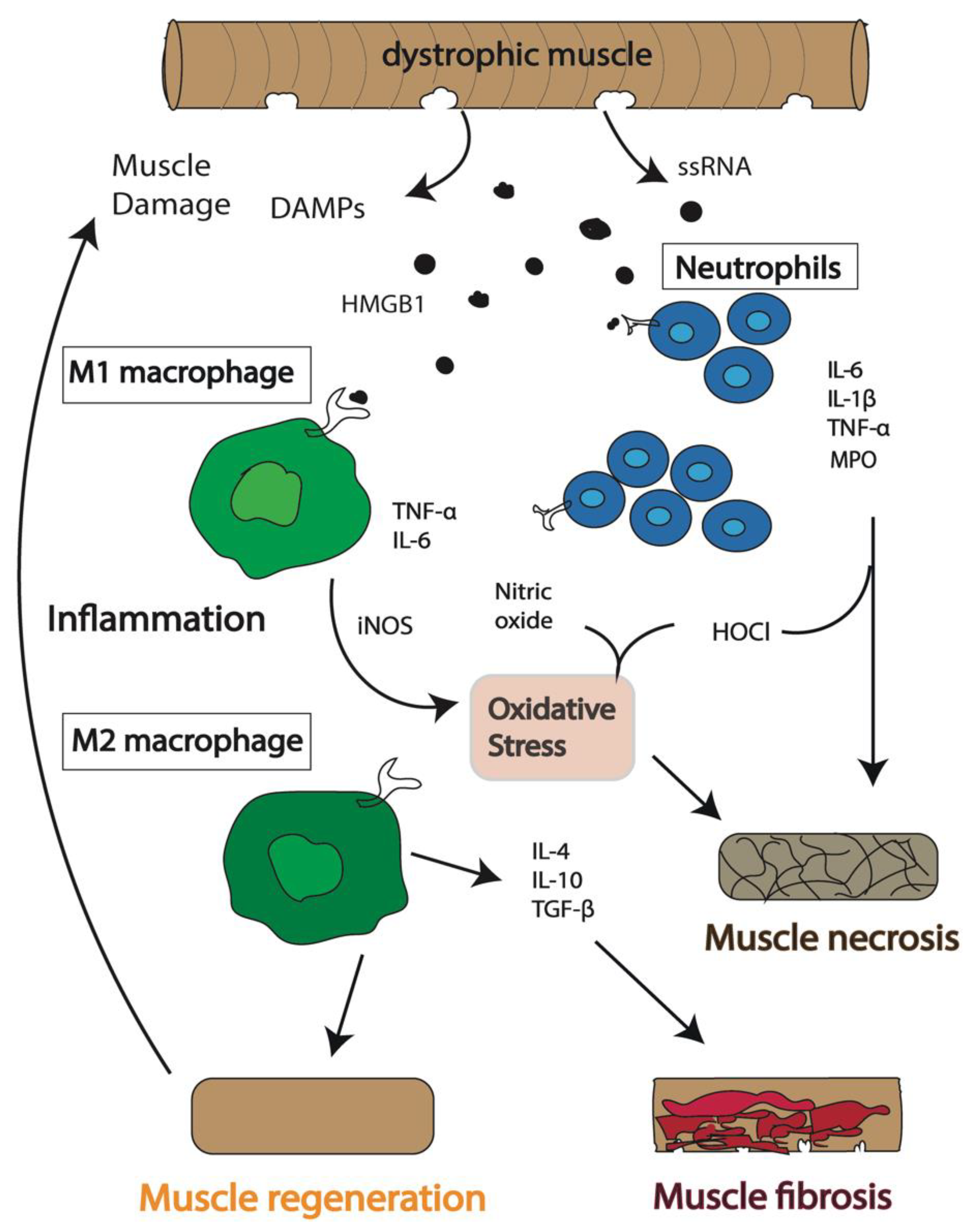
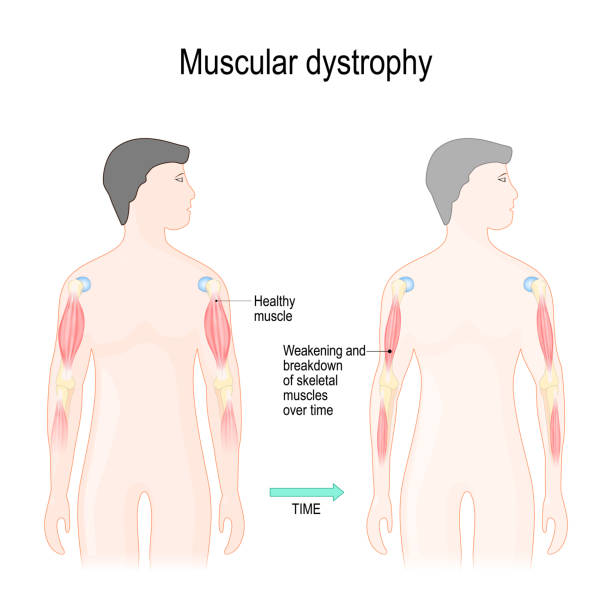
This connection is essential for maintaining muscle fiber integrity during contraction and relaxation. In DMD, mutations prevent the production of functional dystrophin, weakening the muscle membrane and making it prone to damage.
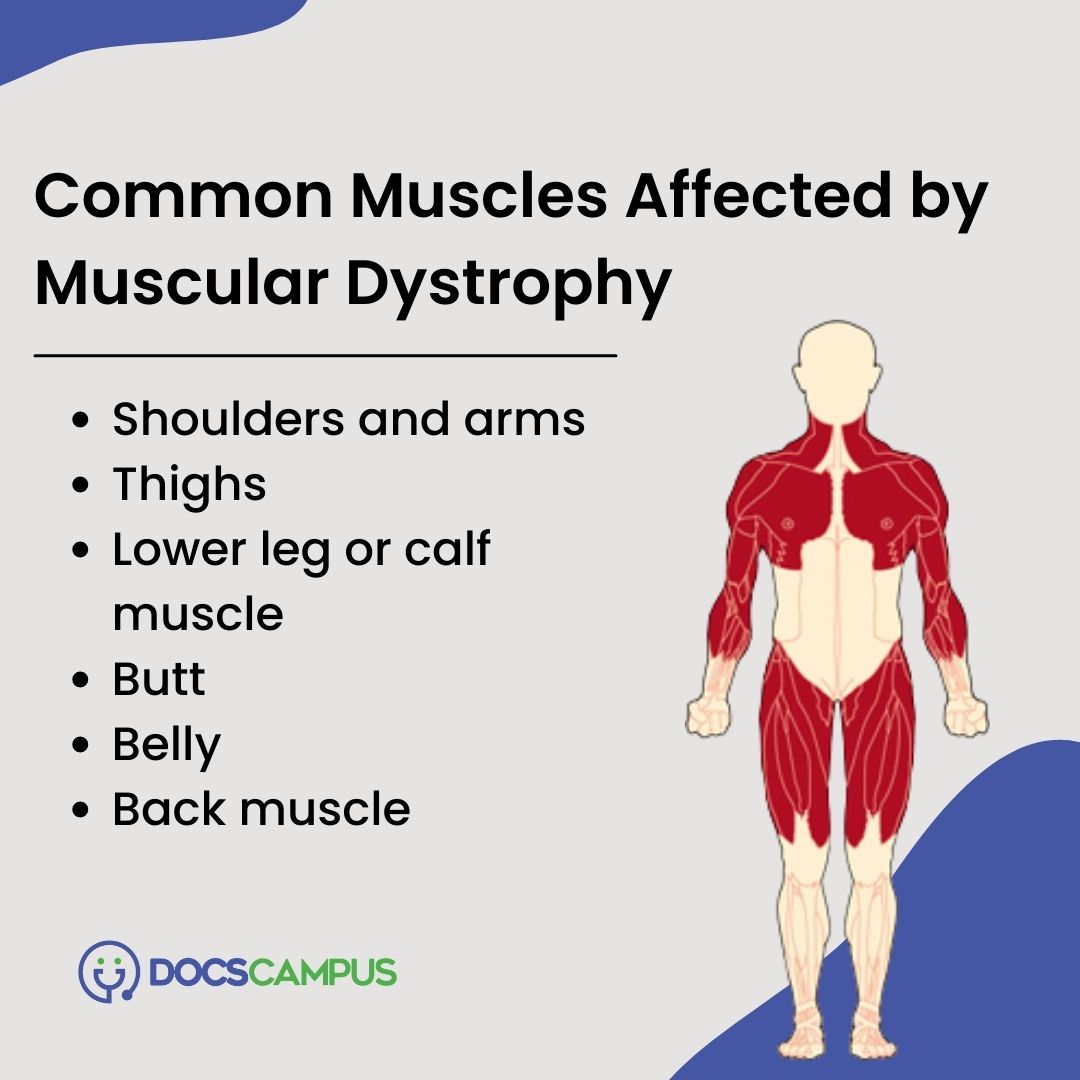
Over time, this repeated damage leads to muscle wasting and the progressive weakness characteristic of the disease.
Types of Mutations
Several types of genetic mutations can disrupt dystrophin production.
The most common are deletions, where sections of the gene are missing. Other mutations include duplications, where sections are repeated, and point mutations, which are single-base changes in the DNA sequence.
These mutations result in either a complete absence of dystrophin or a non-functional, truncated version of the protein.
The severity of DMD symptoms can sometimes correlate with the specific type of mutation. However, this is not always a straightforward relationship.
Some mutations might lead to a slightly shorter, but partially functional, protein, resulting in a milder form of the disease called Becker Muscular Dystrophy (BMD).
Inheritance Pattern: X-Linked Recessive
DMD is inherited in an X-linked recessive pattern. This means that the gene responsible for the condition is located on the X chromosome, and a male needs only one copy of the mutated gene to develop the disease.
Females, with two X chromosomes, typically require two copies of the mutated gene to exhibit DMD symptoms. However, females with one copy are considered carriers.
These carriers usually do not show symptoms, but they have a 50% chance of passing the mutated gene to their children.
If a carrier mother has a son, there's a 50% chance he will inherit the mutated X chromosome and develop DMD. If she has a daughter, there's a 50% chance she will become a carrier.
For affected fathers, all their daughters will become carriers, while none of their sons will inherit the DMD-causing mutation.
Spontaneous mutations can also occur, meaning that a child can develop DMD even if there is no family history of the disease. Approximately one-third of DMD cases arise from such spontaneous mutations.
Diagnosis and Impact
DMD is typically diagnosed in early childhood, often between the ages of 2 and 5. Early signs include delayed motor milestones, such as difficulty walking or climbing stairs.
Diagnostic tests include blood tests to measure creatine kinase (CK) levels, which are elevated in individuals with muscle damage. Genetic testing is then performed to confirm the diagnosis by identifying the specific mutation in the dystrophin gene.
Muscle biopsies can also be used to examine the amount and quality of dystrophin protein present in muscle tissue.
"A confirmed diagnosis of DMD can be devastating for families," says Dr. Emily Carter, a leading researcher in neuromuscular disorders. "It’s crucial to provide comprehensive support and access to the latest treatments to improve the quality of life for affected individuals."
The impact of DMD extends beyond the individual diagnosed, affecting families, caregivers, and the healthcare system. The progressive nature of the disease requires ongoing medical care, assistive devices, and emotional support.
Current and Future Research
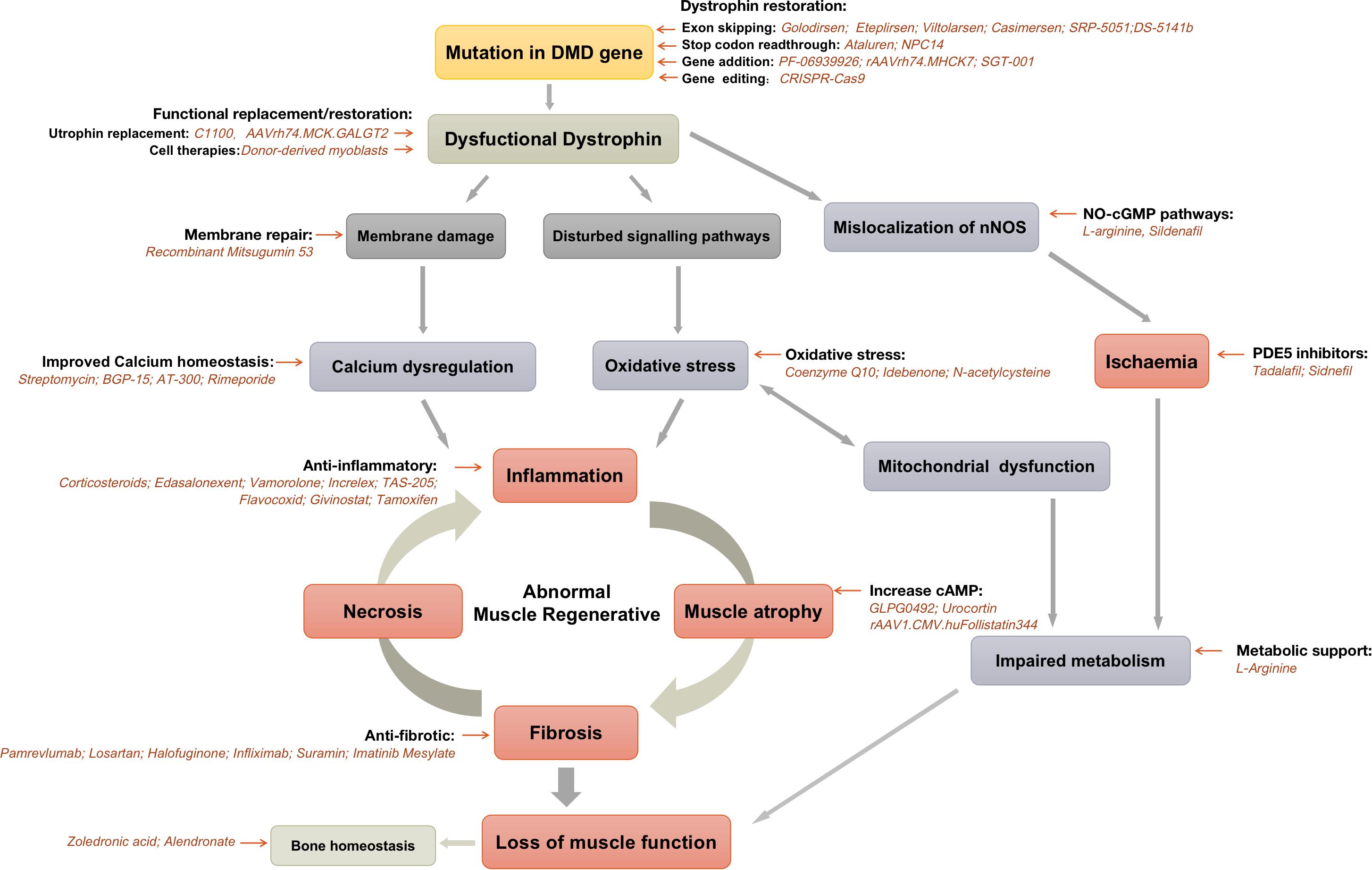
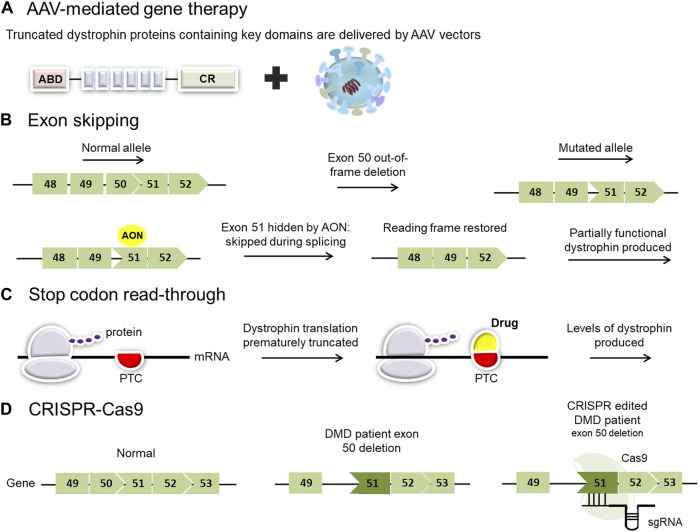
Research efforts are focused on developing therapies that can restore dystrophin production or mitigate the effects of its absence. These include exon skipping therapies, which aim to bypass specific mutations in the dystrophin gene, allowing the production of a shorter but partially functional protein.
Gene therapy is another promising approach, involving the delivery of a functional dystrophin gene into muscle cells. Other research areas include developing drugs that reduce muscle inflammation and promote muscle regeneration.
While a cure for DMD remains elusive, ongoing research provides hope for improving the lives of individuals affected by this debilitating disease.
Understanding the intricate genetic mechanism behind Duchenne Muscular Dystrophy is paramount for developing effective treatments and, ultimately, a cure. Continued research and increased awareness are essential to support those living with DMD and their families.