What Distinguishes One Amino Acid From Another

Life, at its most fundamental level, is a symphony of molecular interactions. The building blocks of this symphony are amino acids, the fundamental units that assemble into proteins, the workhorses of the cell. But what transforms these seemingly simple molecules into the diverse orchestra of life, capable of catalyzing reactions, transporting molecules, and forming structural scaffolds? The answer lies in their subtle yet profound differences: the R-group, also known as the side chain.
Understanding the distinctions between amino acids is crucial not only for comprehending the intricate mechanisms of biology but also for advancing fields like medicine, biotechnology, and materials science. It unlocks possibilities for designing novel drugs, engineering proteins with specific functions, and creating new bio-based materials. This article delves into the key differentiating factor between amino acids – the R-group – exploring its structure, properties, and its profound impact on protein structure and function.
The Universal Amino Acid Structure
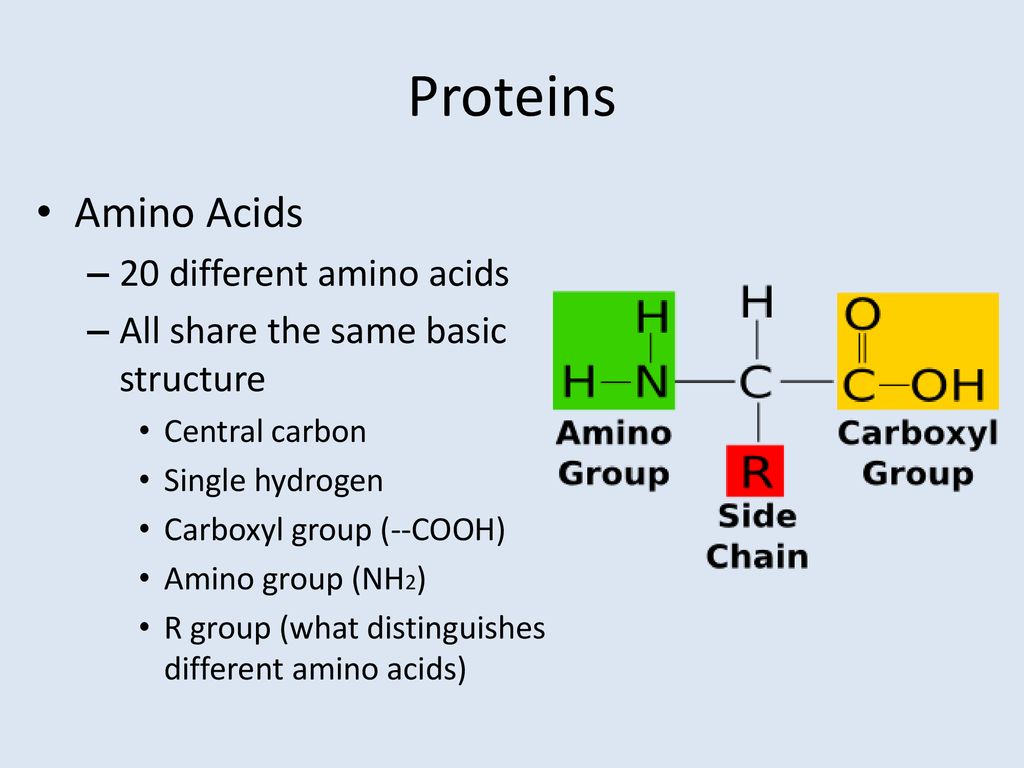
All 20 standard amino acids share a common core structure. This includes a central carbon atom (the alpha carbon), an amino group (-NH2), a carboxyl group (-COOH), and a hydrogen atom (-H). These are all covalently bonded to the alpha carbon.
It is the fourth substituent attached to the alpha carbon – the R-group – that defines each amino acid's unique identity. Each R-group possesses distinct chemical properties, contributing to the vast diversity of protein structure and function.
Decoding the R-Group: Size, Shape, and Charge
R-groups vary dramatically in size, shape, charge, hydrophobicity, and reactivity. These differences are what dictate how an amino acid interacts with other molecules, including other amino acids, water, and various cellular components.
Amino acids are often categorized based on the properties of their R-groups. These broad categories are nonpolar, polar uncharged, positively charged (basic), and negatively charged (acidic). Each category dictates the behavior of the amino acid in a protein structure.
Nonpolar Amino Acids: Hydrophobic Interactions
Nonpolar R-groups are characterized by their aversion to water, often described as hydrophobic. These R-groups consist primarily of carbon and hydrogen atoms, forming nonpolar covalent bonds.
Amino acids like alanine, valine, leucine, isoleucine, phenylalanine, tryptophan, and methionine fall into this category. They tend to cluster together within the interior of proteins, away from the aqueous environment, stabilizing protein structure through hydrophobic interactions. Proline, although technically an imino acid, is often grouped with the nonpolar amino acids due to its unique ring structure and its effect on protein conformation.
Polar Uncharged Amino Acids: Hydrogen Bonding
Polar uncharged R-groups contain atoms that create a dipole moment, allowing them to form hydrogen bonds with water and other polar molecules. While they are not charged at physiological pH, they are hydrophilic, meaning they are attracted to water.
Serine, threonine, cysteine, tyrosine, asparagine, and glutamine belong to this group. These amino acids frequently participate in hydrogen bonding networks within proteins and at protein surfaces, playing crucial roles in enzyme catalysis and protein-ligand interactions.
The presence of hydroxyl (-OH) groups in serine, threonine, and tyrosine also makes them targets for phosphorylation, a critical regulatory modification in cells. Cysteine's sulfhydryl (-SH) group can form disulfide bonds with other cysteine residues, cross-linking different parts of a protein and stabilizing its three-dimensional structure.
Positively Charged (Basic) Amino Acids: Ionic Bonds
Positively charged R-groups carry a net positive charge at physiological pH. This charge arises from the presence of basic functional groups in the side chain.
Lysine, arginine, and histidine are the three positively charged amino acids. They are generally located on the surface of proteins, where they can interact with negatively charged molecules or participate in ionic bonds.
Histidine is unique because its imidazole ring has a pKa near physiological pH, meaning it can be protonated or deprotonated depending on the local environment. This property makes histidine an important catalytic residue in many enzymes.
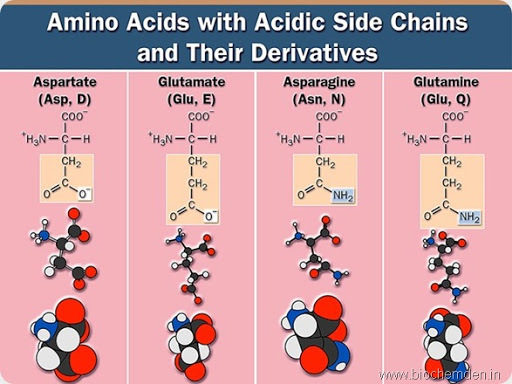
Negatively Charged (Acidic) Amino Acids: Salt Bridges
Negatively charged R-groups carry a net negative charge at physiological pH. These groups contain acidic functional groups, such as carboxyl groups.
Aspartic acid (aspartate) and glutamic acid (glutamate) are the two negatively charged amino acids. Like the positively charged amino acids, they are typically found on the surface of proteins and can participate in ionic bonds, also called salt bridges, with positively charged residues.
Impact on Protein Structure and Function
The specific sequence of amino acids in a polypeptide chain, along with the properties of their R-groups, dictates the protein's three-dimensional structure. This structure, in turn, determines the protein's function.
The primary structure is the linear sequence of amino acids. The secondary structure refers to local folding patterns like alpha-helices and beta-sheets, stabilized by hydrogen bonds between the backbone atoms. The tertiary structure describes the overall three-dimensional shape of a single polypeptide chain, influenced by interactions between R-groups, including hydrophobic interactions, hydrogen bonds, ionic bonds, and disulfide bonds.
Finally, quaternary structure refers to the arrangement of multiple polypeptide chains in a multi-subunit protein. The interactions between the subunits are also governed by the properties of the amino acid R-groups.
Beyond the 20: Non-Standard Amino Acids
While the 20 standard amino acids are the most common building blocks of proteins, there are also numerous non-standard amino acids that are incorporated into proteins after translation. These modifications can introduce further diversity and fine-tune protein function.
Examples include hydroxyproline, found in collagen, and selenocysteine, a rare amino acid that contains selenium instead of sulfur. These non-standard amino acids often play specialized roles in particular proteins.
Future Directions and Applications
Understanding the distinctions between amino acids is fueling advancements in various fields. Protein engineering relies on manipulating amino acid sequences to create proteins with enhanced or novel functions.
In drug discovery, understanding how amino acid residues in drug targets interact with potential drug molecules is essential for rational drug design. Furthermore, synthetic biology utilizes the knowledge of amino acid properties to create artificial proteins and even expand the genetic code.
The study of amino acid properties continues to be a central focus in biological research, promising further breakthroughs in our understanding of life and the development of innovative technologies.


%2C+a+carboxyl+group+(COOH)%2C+and+a+side+group+(R).jpg)