What Does Solid Helium Look Like

Imagine peering into a cryostat, a specialized container designed to maintain extremely low temperatures. Within, instead of the swirling liquids you might expect, sits something extraordinary. It’s not quite solid in the conventional sense, not like ice or rock. Instead, it resembles a shimmering, translucent block, an ethereal substance that appears both fragile and incredibly strong – this is solid helium, a state of matter so unique it defies everyday intuition.
The existence of solid helium, a seemingly impossible substance, challenges our understanding of matter's fundamental states. Understanding its properties allows scientists to probe the very nature of quantum mechanics and explore the limits of physical possibility. This seemingly esoteric research has implications for fields ranging from materials science to cosmology, offering a glimpse into the extreme conditions that govern the universe.
A Brief History of Helium and its Oddities
Helium, the second most abundant element in the universe, was first discovered in 1868 by French astronomer Pierre Janssen during a solar eclipse. Observing spectral lines emitted from the sun, he identified a new element, later named after the Greek word for sun, "helios". It wasn’t until 1908 that Dutch physicist Heike Kamerlingh Onnes, who also achieved the first successful liquefaction of helium, successfully solidified it.
This feat was no small achievement. Unlike most substances that solidify upon cooling, helium requires both extremely low temperatures and high pressure. This is because helium atoms are exceptionally light and weakly interacting, resisting the transition to a solid state unless forced to do so by external pressure.
Even at absolute zero, the lowest temperature theoretically possible, helium's atoms possess enough kinetic energy to prevent them from locking into a rigid lattice structure under normal pressure. Only by squeezing the atoms together, increasing the density and therefore the interatomic forces, can helium be coerced into solidifying.
What Does Solid Helium *Really* Look Like?
Visualizing solid helium is tricky. It’s not simply a frozen block of the gas you find in party balloons. It's a complex quantum solid, and its appearance depends on the specific conditions under which it's formed.
In many cases, solid helium appears translucent, almost glass-like, sometimes with a slightly bluish tint due to Rayleigh scattering, the same phenomenon that makes the sky blue. The clarity varies depending on the crystal structure. If the helium solidifies rapidly, it can form a polycrystalline structure with numerous small crystals, giving it a more opaque, milky appearance.
However, if it's grown slowly under controlled conditions, it can form large, single crystals that are remarkably clear. Imagine a block of ice, but even more pristine and ethereal, shimmering with a faint light. That’s a good mental image, although it’s important to remember the quantum weirdness that lurks beneath the surface.
Two Forms: Helium-4 and Helium-3
It's important to distinguish between the two stable isotopes of helium: Helium-4 and Helium-3. Helium-4, the far more abundant isotope, has two protons and two neutrons in its nucleus. Helium-3 has two protons and only one neutron. This seemingly small difference has profound consequences for their behavior at low temperatures.
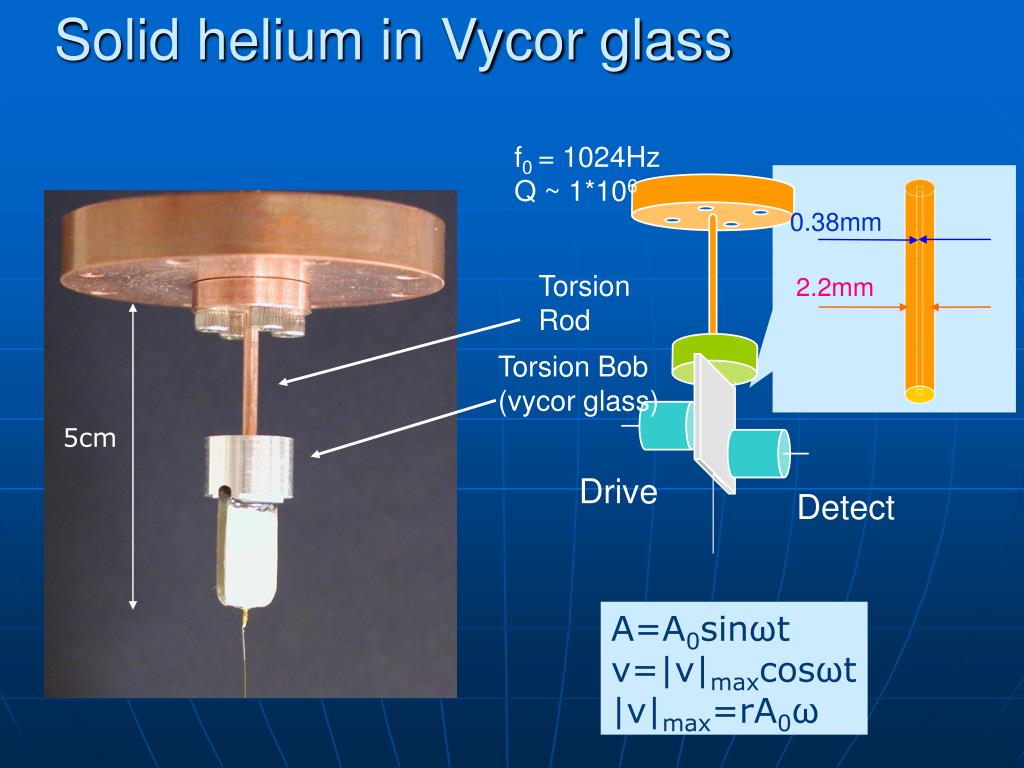
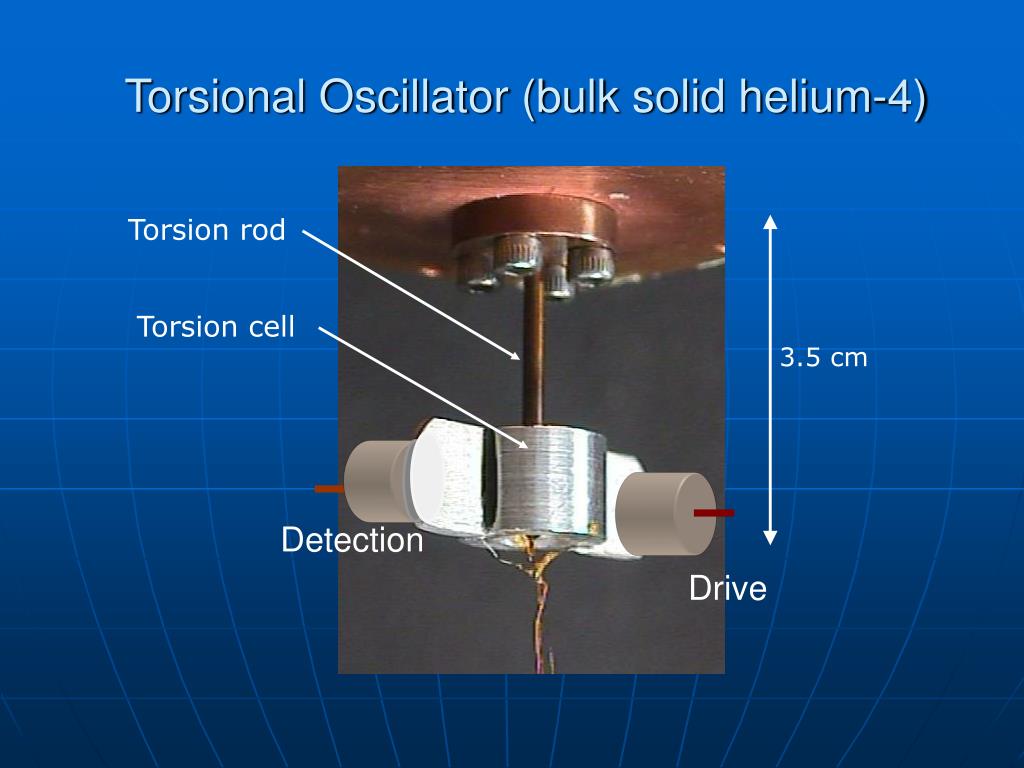
Helium-4 becomes a superfluid at around 2.17 Kelvin (-270.98 °C). This means it flows without any viscosity, defying gravity and climbing up the sides of containers. Solid helium-4 exists under high pressure and exhibits fascinating properties like supersolidity.
Helium-3, on the other hand, requires even lower temperatures to exhibit superfluidity (around 0.0025 Kelvin). Solid helium-3 also has different magnetic properties compared to helium-4 due to the unpaired neutron in its nucleus.
Supersolidity: A State of Matter that Defies Intuition
One of the most intriguing and debated properties of solid helium is the possibility of supersolidity. This theoretical state of matter would possess the seemingly contradictory properties of both a solid and a superfluid. In other words, it would be a solid capable of flowing without any resistance.
The idea of supersolidity was first proposed by Alexander Andreev and Ilya Lifshitz in 1969. They suggested that under certain conditions, a fraction of the atoms in a solid could condense into a superfluid state, allowing the solid to flow without friction. Think of it as a solid with a built-in, frictionless channel for atoms to move through.
While the experimental evidence for supersolidity in solid helium is complex and has been subject to much debate, recent research continues to provide tantalizing clues. It's a field where new discoveries are constantly being made, pushing the boundaries of our understanding of condensed matter physics. The search for definitive proof of supersolidity continues to drive innovative experimental techniques and theoretical models.
Why Study Solid Helium?
The study of solid helium, especially the search for supersolidity, isn't just an academic exercise. Understanding the behavior of matter at extreme conditions has profound implications for other areas of science and technology. Its quantum properties mean solid helium serves as a laboratory for understanding quantum mechanics at a macroscopic scale.
Knowledge gained from studying solid helium can contribute to the development of new materials with unique properties. This includes materials with enhanced superconductivity or improved energy storage capabilities. This research could influence advances in technologies such as high-speed computing and energy-efficient devices.
Furthermore, the extreme conditions required to create and study solid helium are similar to those found in the interiors of planets and stars. This knowledge provides valuable insights into the formation and evolution of celestial bodies. It helps us understand the composition and behavior of matter under immense pressure and temperature.
The Ongoing Quest
The study of solid helium, particularly the quest to definitively prove the existence of supersolidity, exemplifies the scientific process. It’s a journey filled with unexpected discoveries, rigorous testing, and ongoing refinement of theories. The pursuit of knowledge often leads to more questions than answers.
While the definitive existence of supersolidity in solid helium remains a topic of debate, the research it has inspired has already yielded significant advancements in our understanding of condensed matter physics. It showcases the power of curiosity-driven research to push the boundaries of human knowledge. These advancements are not just theoretical; they also pave the way for technological innovation in areas like materials science and quantum computing.
So, the next time you see a helium balloon, remember that it represents more than just a floating toy. It's a reminder of the extraordinary properties of this element, a substance that can exist in a state so bizarre that it challenges our fundamental understanding of the world around us. It’s a journey into the quantum realm, a world of possibilities as boundless as the universe itself, and it all starts with a shimmering, translucent block of solid helium.