What Is A Dose Limiting Toxicity

In the high-stakes world of medical research, particularly in the development of new cancer therapies, the phrase "dose-limiting toxicity" carries significant weight. It represents a critical juncture in clinical trials, a point where potential benefits of a drug are weighed against unacceptable risks to patient health. The identification of a dose-limiting toxicity (DLT) can halt or significantly alter the course of a drug's development, impacting countless lives and millions of dollars in research funding.
This article delves into the concept of dose-limiting toxicity, exploring its definition, the factors influencing its occurrence, and its profound implications for drug development and patient safety. Understanding DLTs is crucial not only for medical professionals and researchers, but also for patients participating in clinical trials and the wider public seeking to comprehend the complexities of modern medicine.
What is Dose-Limiting Toxicity?
At its core, a dose-limiting toxicity (DLT) is a severe adverse effect of a drug that prevents further dose escalation in a clinical trial. This toxicity is considered unacceptable at a given dose level because it poses a significant threat to the patient's well-being. It’s the primary reason a therapy may be determined unsafe to continue increasing dosage.
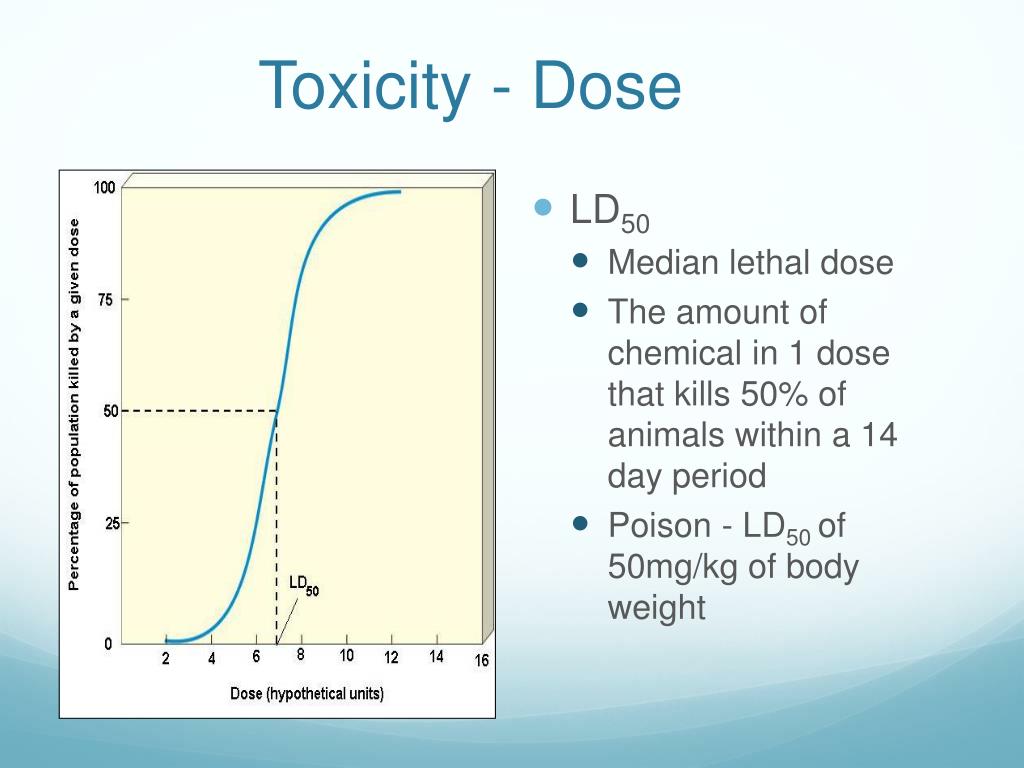
Typically, DLTs are identified during Phase I clinical trials. These trials are designed to determine the maximum tolerated dose (MTD) of a new drug. The goal is to find the highest dose that can be administered without causing unacceptable side effects.
The MTD becomes a critical reference point for subsequent clinical trials.
Factors Influencing Dose-Limiting Toxicities
Several factors can contribute to the occurrence and severity of DLTs. These can be broadly categorized into drug-related factors and patient-related factors. Understanding these influences is crucial for predicting and managing potential toxicities.
Drug-Related Factors
The inherent properties of the drug itself play a significant role. These include its mechanism of action, its pharmacokinetic profile (how the body processes the drug), and its pharmacodynamic profile (how the drug affects the body). For example, a drug that targets a specific protein found in both cancer cells and healthy cells is more likely to cause DLTs.
The drug's formulation and route of administration are also important considerations. Different formulations can affect how quickly the drug is absorbed into the bloodstream. Similarly, intravenous administration may lead to higher peak drug concentrations compared to oral administration, potentially increasing the risk of toxicity.
Patient-Related Factors
A patient's individual characteristics can significantly influence their susceptibility to DLTs. Factors such as age, gender, organ function (especially liver and kidney function), and pre-existing medical conditions can all play a role. Patients with impaired liver or kidney function may be unable to effectively metabolize or excrete the drug, leading to drug accumulation and increased toxicity.
Genetic variations can also affect drug metabolism and response. Pharmacogenomics, the study of how genes affect a person's response to drugs, is increasingly being used to personalize drug dosing and minimize the risk of DLTs.
Prior therapies and concurrent medications can further influence drug response.
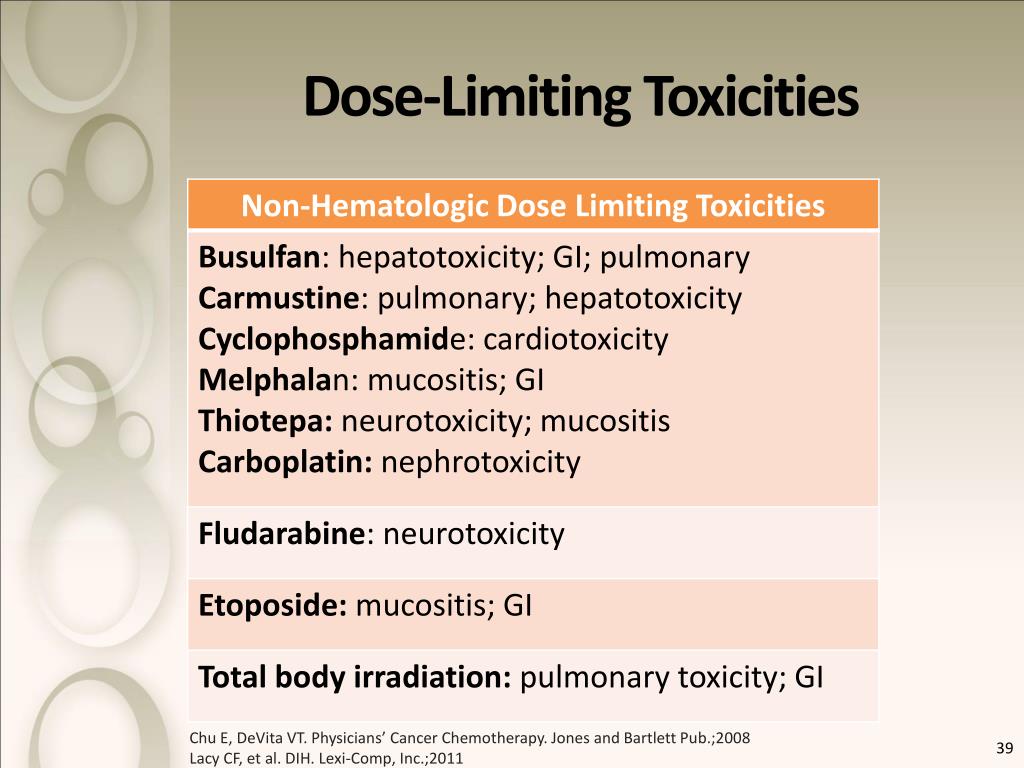
Examples of Common Dose-Limiting Toxicities
DLTs can manifest in various ways, affecting different organ systems. Common examples include hematologic toxicities, gastrointestinal toxicities, and neurological toxicities. These toxicities can significantly impact a patient's quality of life and may require hospitalization or dose reduction.
Hematologic toxicities, such as neutropenia (low white blood cell count) and thrombocytopenia (low platelet count), are frequently observed with chemotherapy drugs. These toxicities increase the risk of infection and bleeding. Gastrointestinal toxicities, such as nausea, vomiting, diarrhea, and mucositis (inflammation of the lining of the digestive tract), are also common. Neurological toxicities can range from mild peripheral neuropathy (nerve damage) to more severe conditions like seizures or encephalopathy (brain dysfunction).
Other DLTs may include cardiotoxicity (damage to the heart), hepatotoxicity (damage to the liver), and nephrotoxicity (damage to the kidneys). The specific DLTs associated with a drug depend on its mechanism of action and its effects on different organ systems.
The Impact of DLTs on Drug Development
The identification of a DLT can have profound implications for the development of a new drug. In some cases, it may lead to the termination of the drug's development. This is particularly true if the DLT is severe, irreversible, or occurs at a dose level that is unlikely to be therapeutically effective.
In other cases, the drug's development may continue, but with modifications. This may involve reducing the dose, altering the dosing schedule, or developing strategies to mitigate the DLT. For example, prophylactic medications may be used to prevent or reduce nausea and vomiting associated with chemotherapy drugs.
The cost of drug development is substantial, and the failure rate is high. DLTs contribute significantly to this failure rate, highlighting the importance of rigorous preclinical testing and careful monitoring during clinical trials.
"DLTs are a crucial consideration in drug development, impacting both patient safety and the economic viability of bringing new therapies to market,"according to a statement from the FDA.
Managing and Mitigating DLTs
Effective management of DLTs requires a multidisciplinary approach. This includes close monitoring of patients for signs and symptoms of toxicity, prompt intervention when toxicities occur, and careful dose adjustments as needed. Healthcare professionals play a crucial role in educating patients about potential side effects and providing support throughout their treatment.
Supportive care measures, such as antiemetics for nausea and vomiting and growth factors to stimulate white blood cell production, can help to mitigate the effects of DLTs. In some cases, dose reductions or treatment interruptions may be necessary to allow the body to recover. The use of biomarkers to predict and monitor toxicity is an area of active research.
Individualized treatment plans, tailored to the patient's specific characteristics and medical history, are essential for minimizing the risk of DLTs. This approach, often referred to as precision medicine, aims to optimize drug dosing and selection based on the patient's unique genetic and clinical profile.
The Future of DLT Research
Research into DLTs is ongoing, with a focus on developing more predictive models, identifying novel biomarkers, and developing strategies to mitigate toxicities. Advances in areas such as pharmacogenomics, nanotechnology, and immunotherapy are offering new opportunities to improve the safety and efficacy of drug therapies.
The development of more targeted therapies, which selectively target cancer cells while sparing healthy cells, is also a promising avenue for reducing the incidence of DLTs. As our understanding of the complex interactions between drugs and the human body grows, we can expect to see further advances in the prevention and management of DLTs. Ultimately, this will lead to safer and more effective treatments for a wide range of diseases.
The ongoing quest to understand and mitigate dose-limiting toxicities remains a critical endeavor. It is a testament to the commitment of researchers, clinicians, and patients to push the boundaries of medical science while prioritizing patient safety and well-being.






.jpg)