What Is The Approved Indication For Acoramidis

FDA Approves Acoramidis for Rare Heart Condition: Acoramidis receives accelerated approval to treat transthyretin amyloid cardiomyopathy (ATTR-CM), offering a new hope for patients with this life-threatening disease.
This approval marks a significant advancement in the treatment landscape for ATTR-CM, a condition characterized by the buildup of amyloid protein in the heart, leading to heart failure.
FDA Greenlights Acoramidis: What You Need to Know
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to acoramidis, a medication developed by Eidos Therapeutics, now a subsidiary of BridgeBio Pharma.
This approval is specifically for the treatment of adult patients with transthyretin amyloid cardiomyopathy (ATTR-CM).
Targeting ATTR-CM: A Breakdown
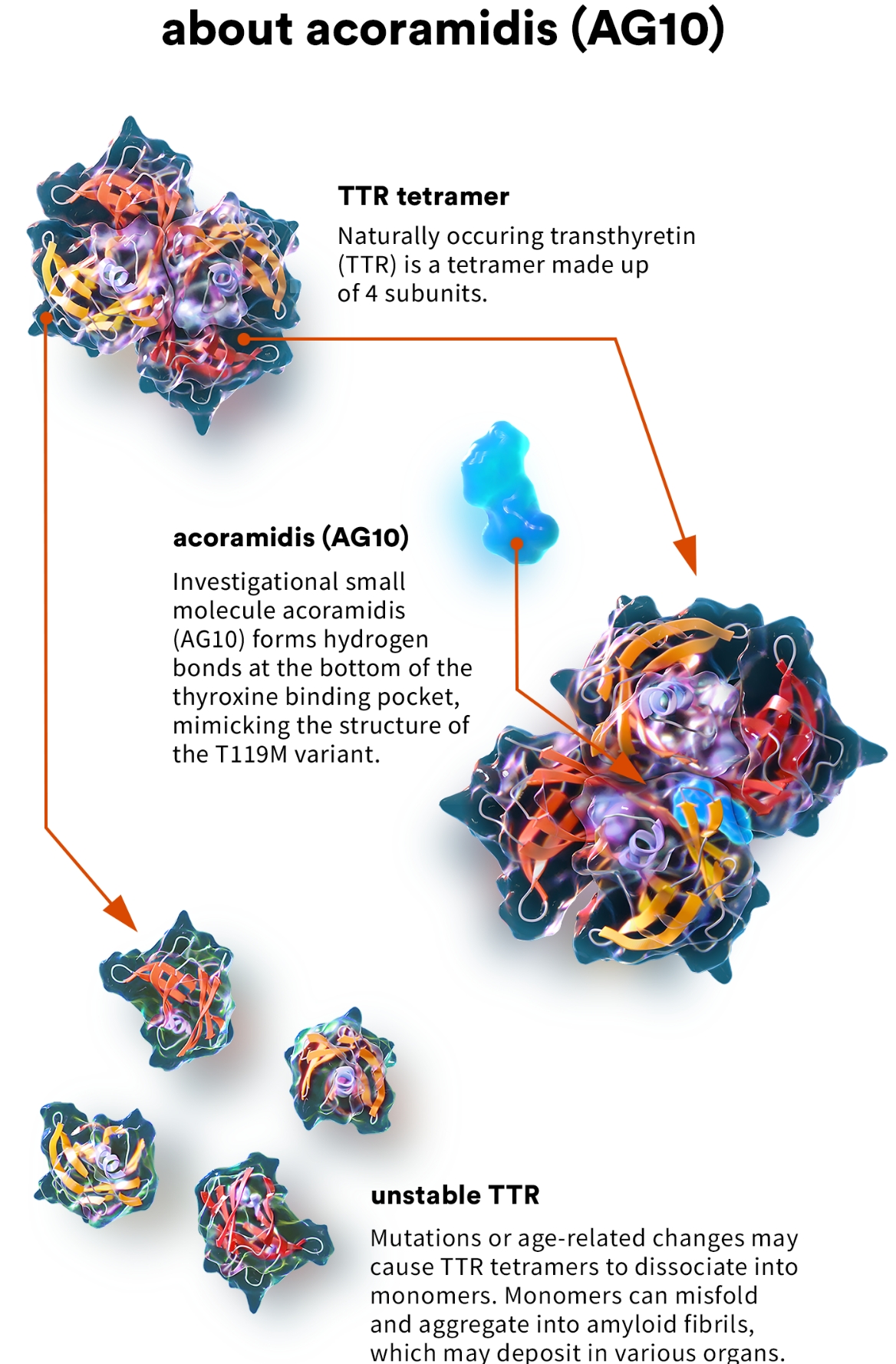
ATTR-CM is a rare and often underdiagnosed condition where misfolded transthyretin (TTR) protein accumulates in the heart.
This accumulation causes the heart muscle to stiffen, hindering its ability to pump blood effectively, ultimately leading to heart failure and death.
There are two main types of ATTR-CM: hereditary (hATTR) and wild-type (wtATTR).
Hereditary ATTR results from mutations in the TTR gene, while wild-type ATTR occurs without a known genetic mutation and is associated with aging.
The Acoramidis Solution: How It Works
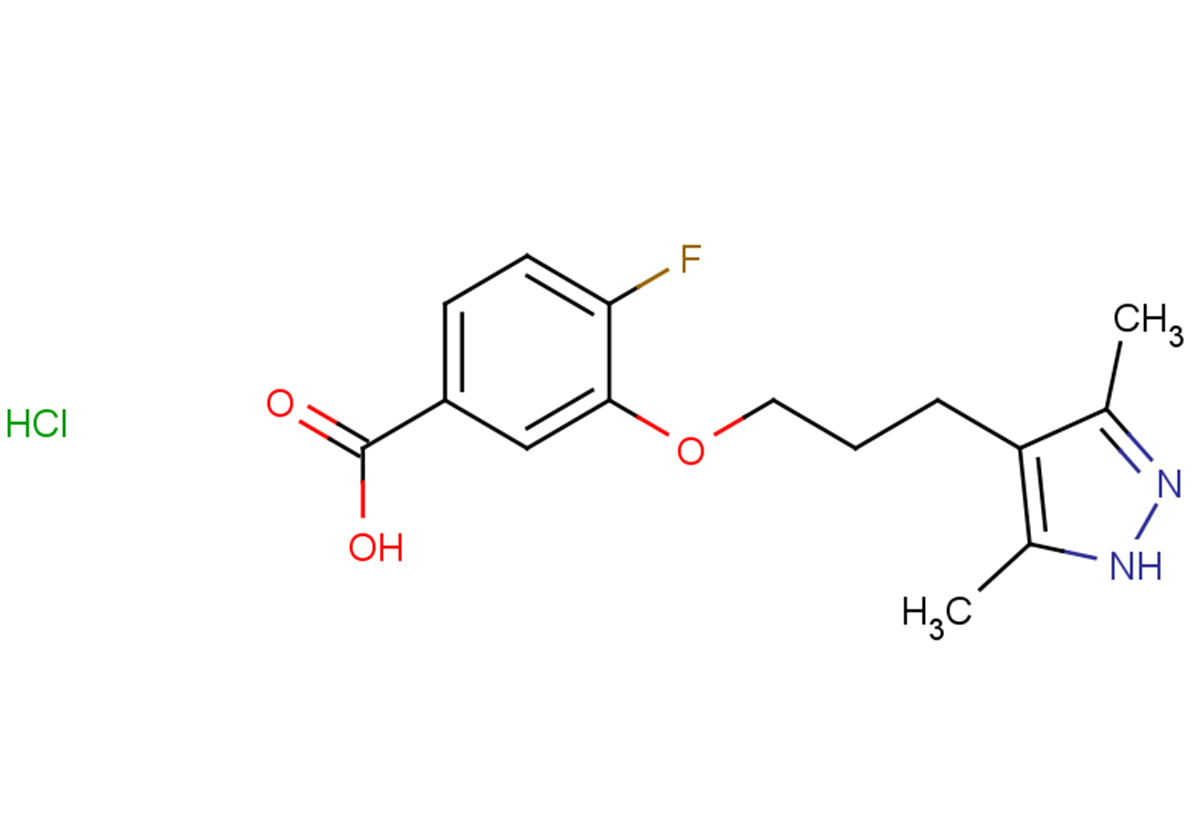
Acoramidis is a TTR stabilizer.
This means it works by binding to and stabilizing the TTR protein, preventing it from misfolding and aggregating in the heart.
By stabilizing the TTR protein, acoramidis aims to slow or halt the progression of ATTR-CM.
Clinical Trial Data: The Basis for Approval
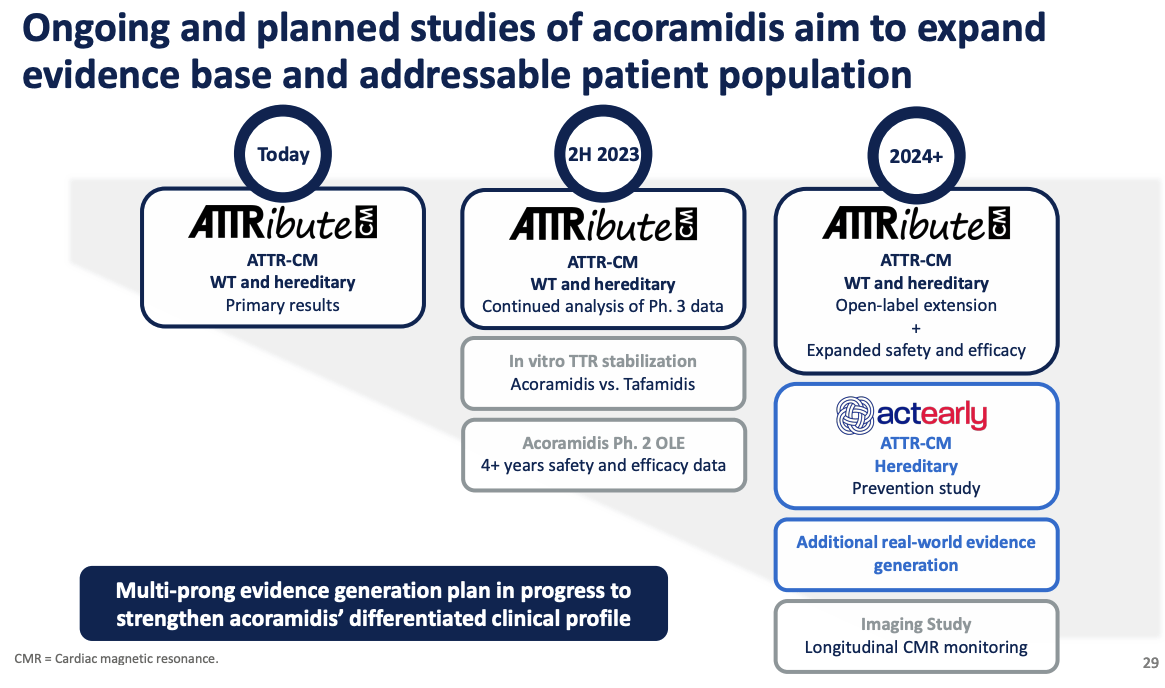
The FDA's accelerated approval of acoramidis is based on data from the ATTRibute-CM clinical trial.
This trial evaluated the efficacy and safety of acoramidis in patients with ATTR-CM.
The results demonstrated that acoramidis significantly improved survival and reduced cardiovascular hospitalizations compared to placebo.
Specifically, the trial met its primary endpoint, demonstrating a statistically significant improvement in the composite outcome of all-cause mortality and cardiovascular hospitalizations.
Accelerated Approval: A Path to Patients
It is important to note that this is an accelerated approval.
This means that continued approval may be contingent upon verification and description of clinical benefit in a confirmatory trial.
BridgeBio Pharma is currently conducting further studies to confirm the clinical benefit of acoramidis.
Who Benefits: Patient Population
Acoramidis is indicated for adult patients diagnosed with ATTR-CM.
This includes both hereditary and wild-type forms of the disease.
Patients experiencing symptoms of heart failure due to ATTR-CM are potential candidates for treatment with acoramidis.
When and Where: Availability and Access
Details regarding the precise launch date and availability of acoramidis are still being finalized by BridgeBio Pharma.
The company is working to ensure that the medication is accessible to patients who need it as quickly as possible.
Patients should consult with their healthcare providers to determine if acoramidis is an appropriate treatment option for them and to understand the potential risks and benefits.
Next Steps: Ongoing Developments
BridgeBio Pharma will continue to work with the FDA to fulfill the requirements for full approval of acoramidis.
This includes ongoing data collection and analysis from the ATTRibute-CM trial and other studies.
The company is also committed to providing support and resources to patients and healthcare professionals involved in the treatment of ATTR-CM.
Further updates regarding the availability and pricing of acoramidis will be provided by BridgeBio Pharma in the coming weeks.
The medical community anticipates further data releases and presentations that will elaborate on the clinical trial results, including subgroup analyses.
Patients and physicians are encouraged to stay informed about the latest developments regarding acoramidis and ATTR-CM treatment.