What Maintains The Secondary Structure Of A Protein
Proteins, the workhorses of our cells, carry out a vast array of functions crucial for life, from catalyzing biochemical reactions to transporting molecules and providing structural support. Understanding how proteins attain and maintain their intricate three-dimensional structures is fundamental to comprehending their function and developing new therapies for diseases linked to protein misfolding.
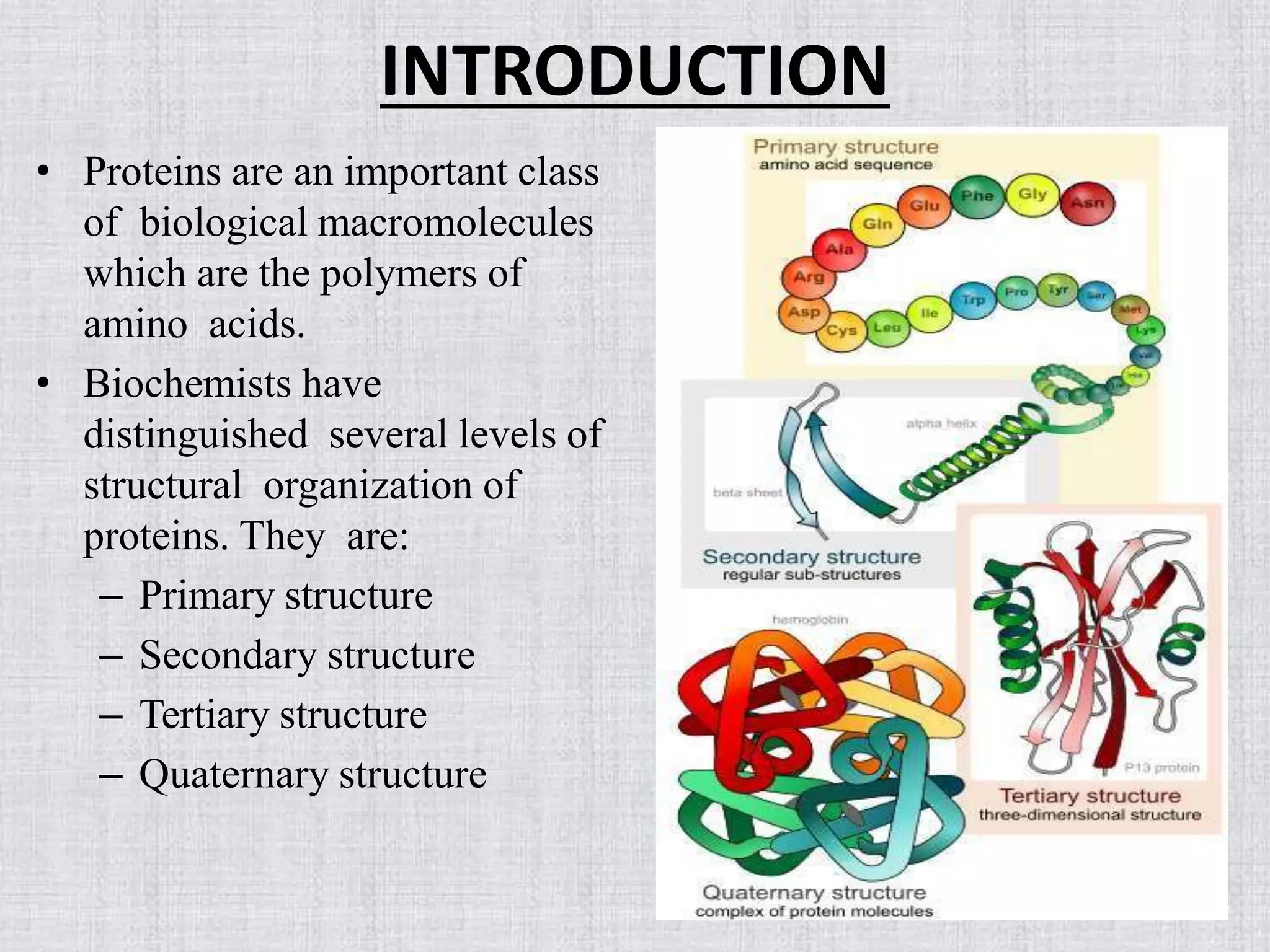
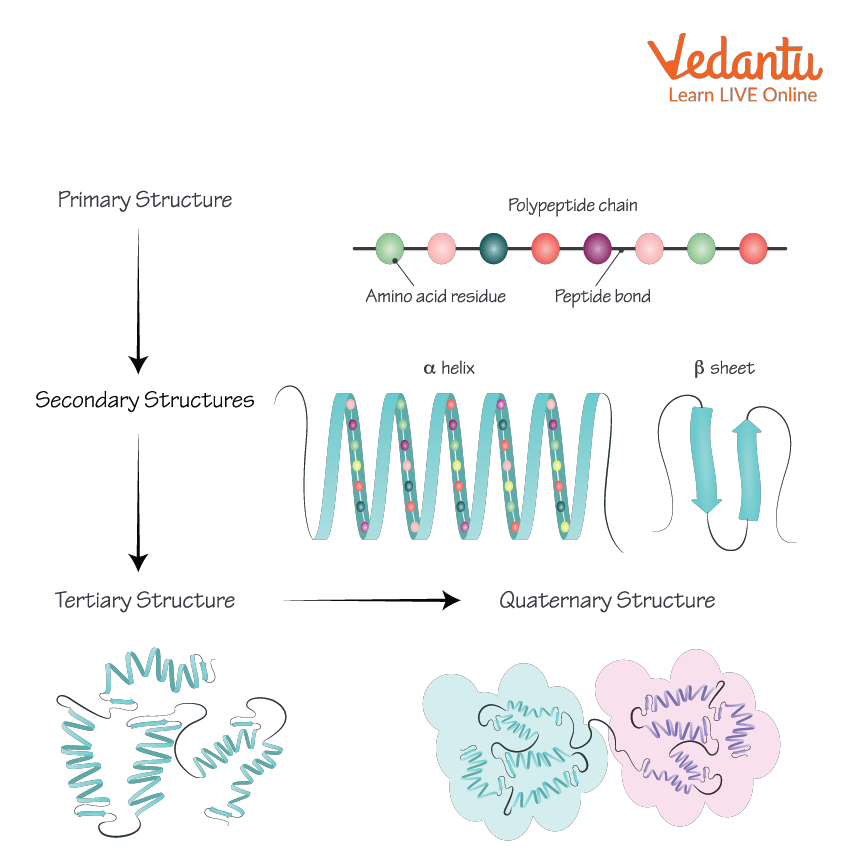
At the heart of this structural organization lies the secondary structure, a level of protein architecture defined by recurring patterns of hydrogen bonds along the polypeptide backbone. These patterns, primarily the alpha-helix and the beta-sheet, provide the initial folding framework upon which more complex structures are built. What exactly maintains these vital secondary structures, and why is it important?
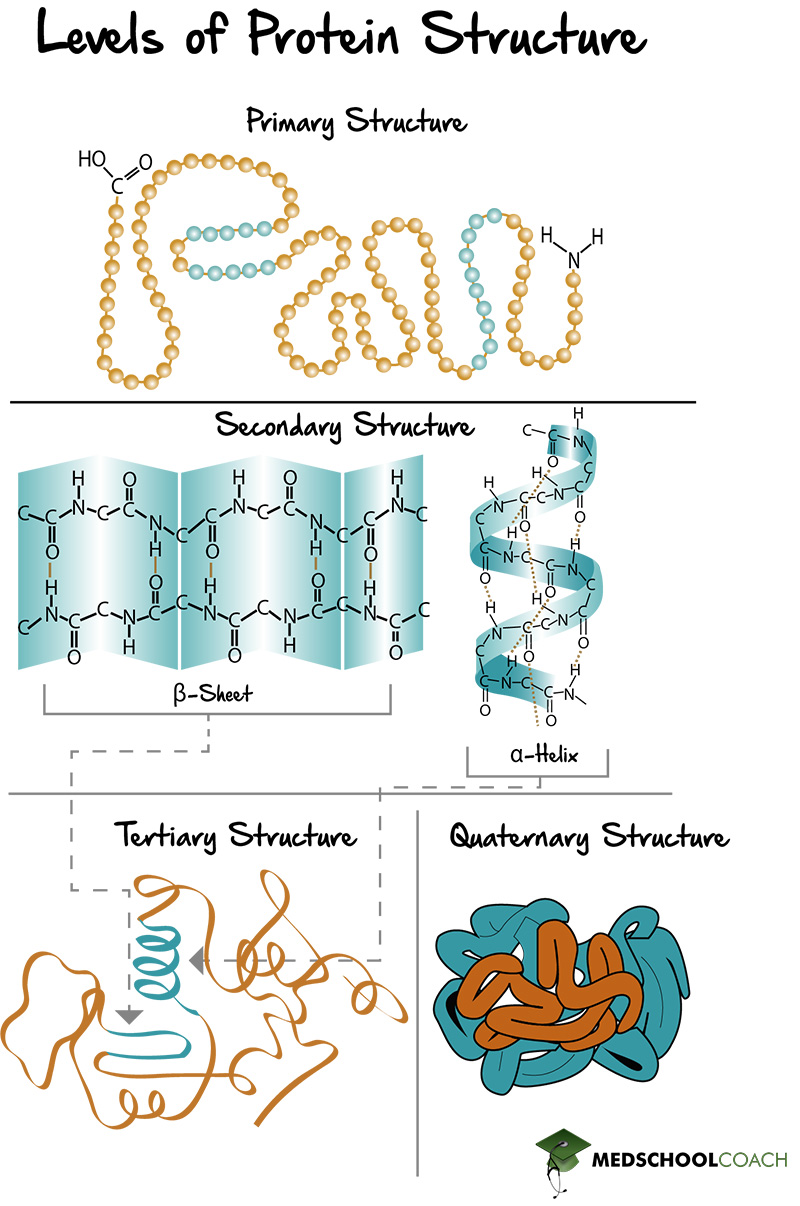
The secondary structure of a protein refers to the local conformation of the polypeptide backbone. It's the arrangement of the protein chain into regular structures such as the alpha-helix and beta-sheet. Understanding the forces that stabilize these arrangements is crucial to understanding protein function.
The Hydrogen Bond: The Primary Stabilizer
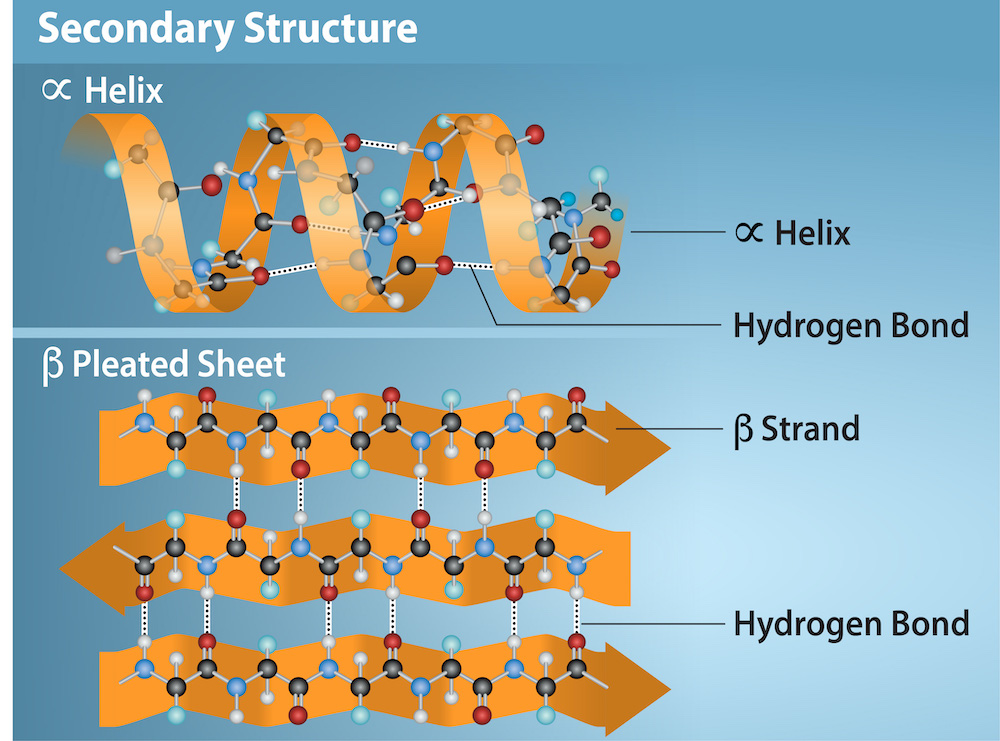
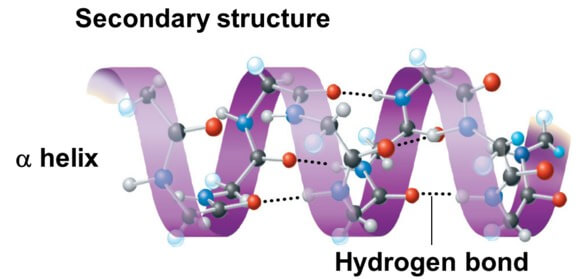
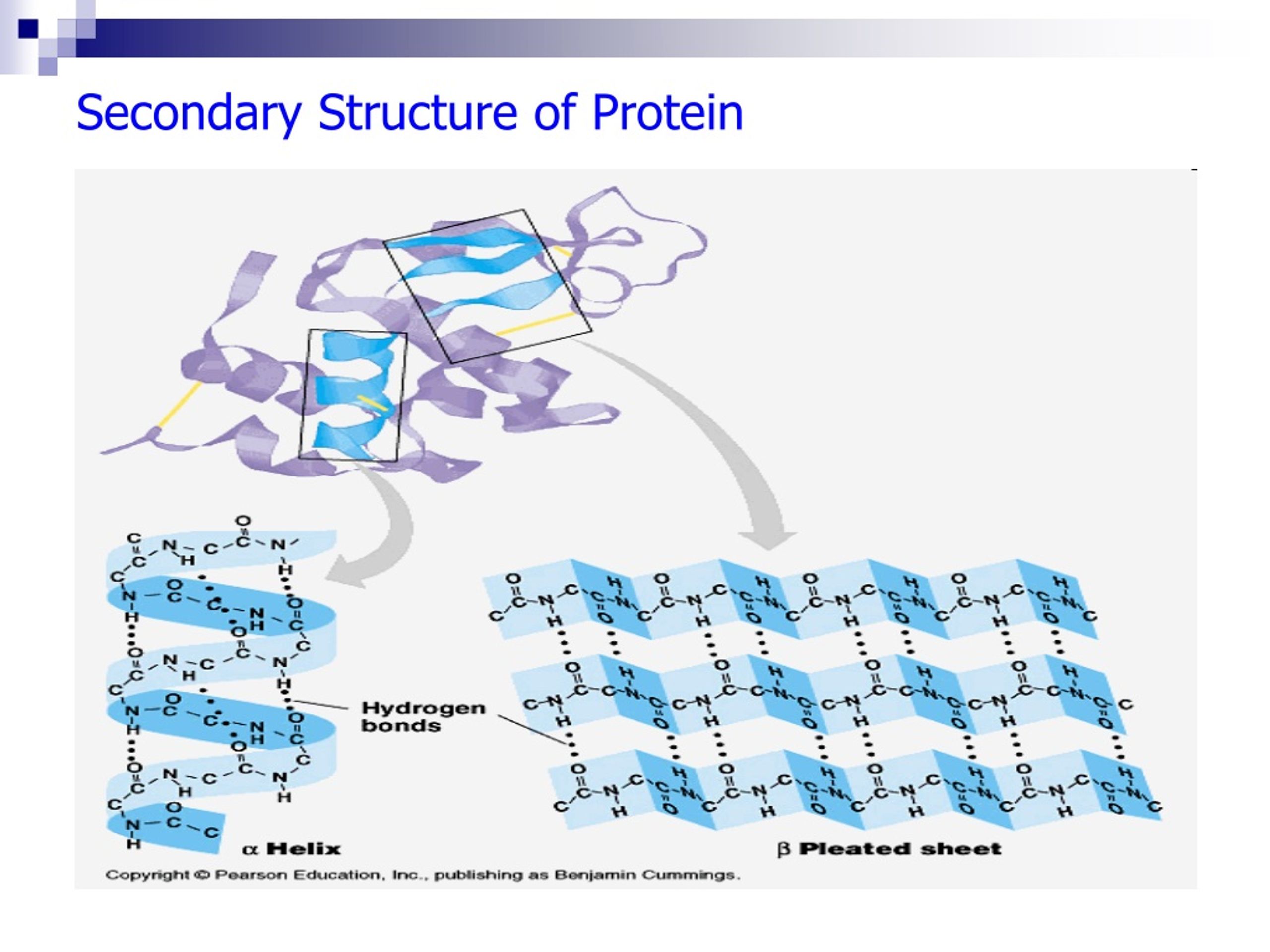
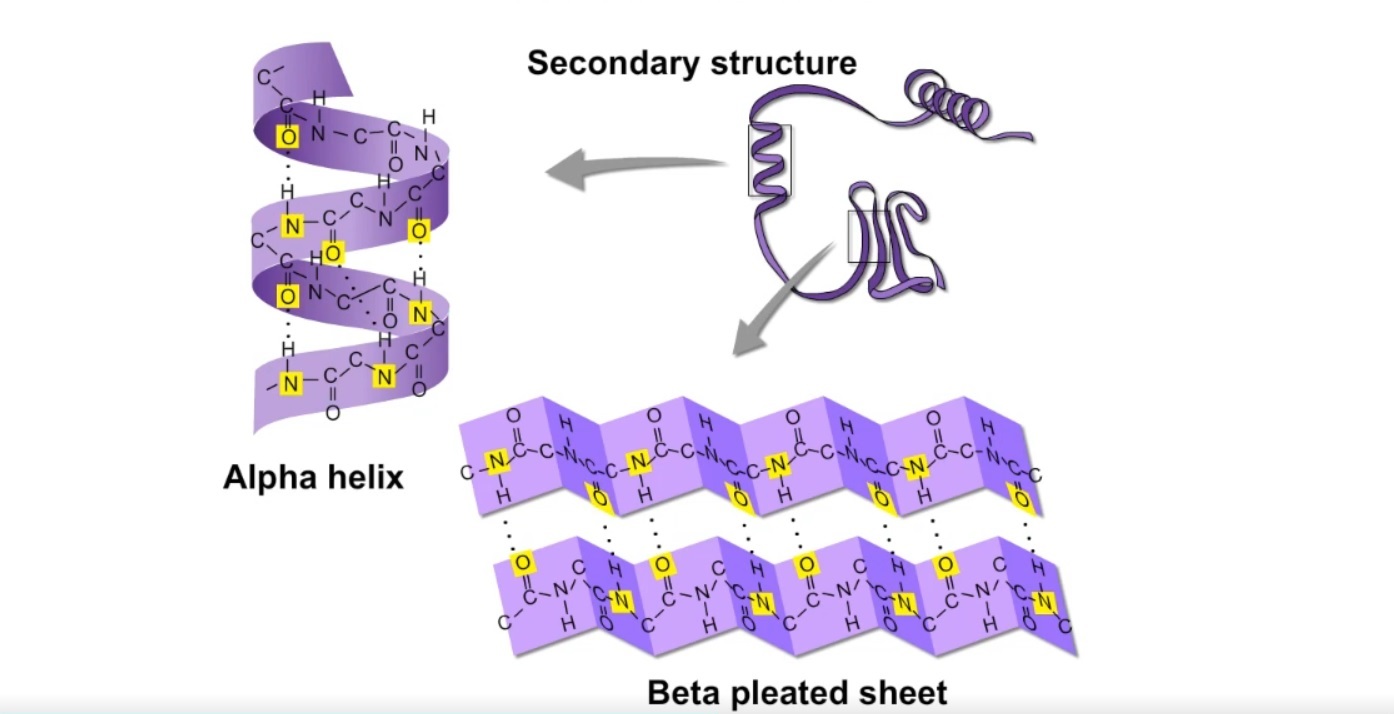
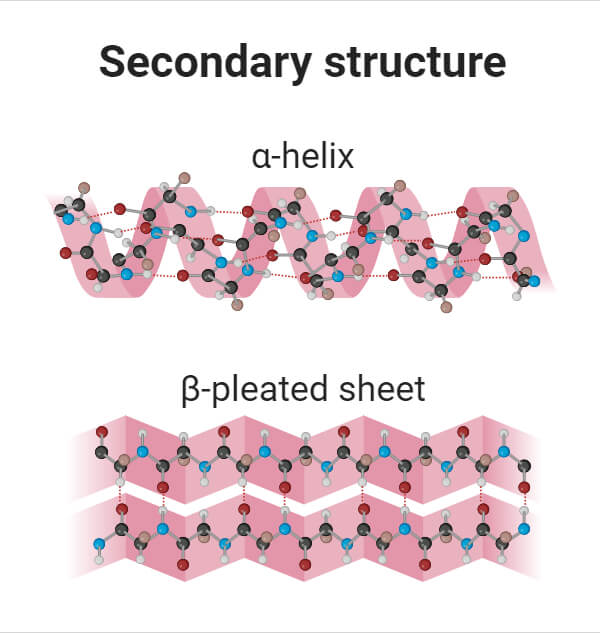
The secondary structure of a protein is primarily maintained by hydrogen bonds. These bonds form between the carbonyl oxygen (C=O) of one amino acid and the amide hydrogen (N-H) of another amino acid in the polypeptide chain.
It is important to note that these hydrogen bonds are formed between the atoms of the peptide backbone itself, not the side chains (R-groups) of the amino acids. This distinction highlights that secondary structure is a fundamental property of the polypeptide backbone, independent of specific amino acid sequence.
The specific pattern of hydrogen bonds dictates whether an alpha-helix or a beta-sheet will form.
Alpha-Helices: A Tightly Wound Coil
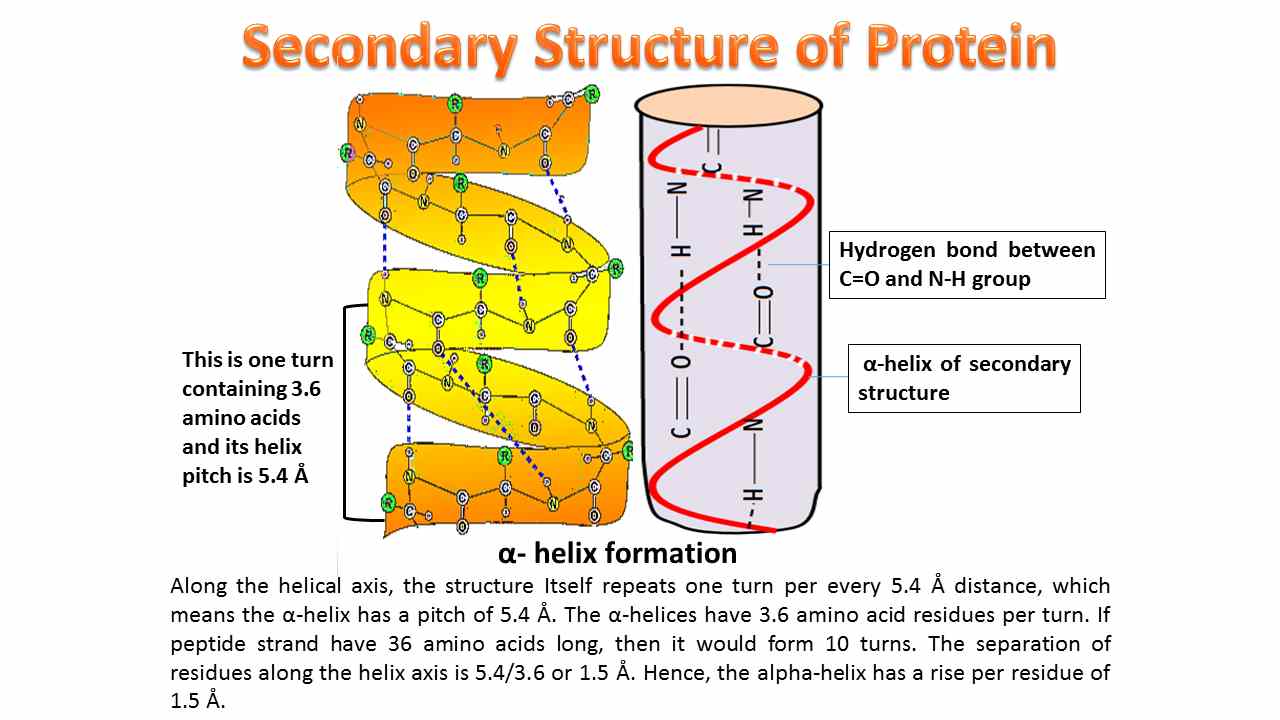
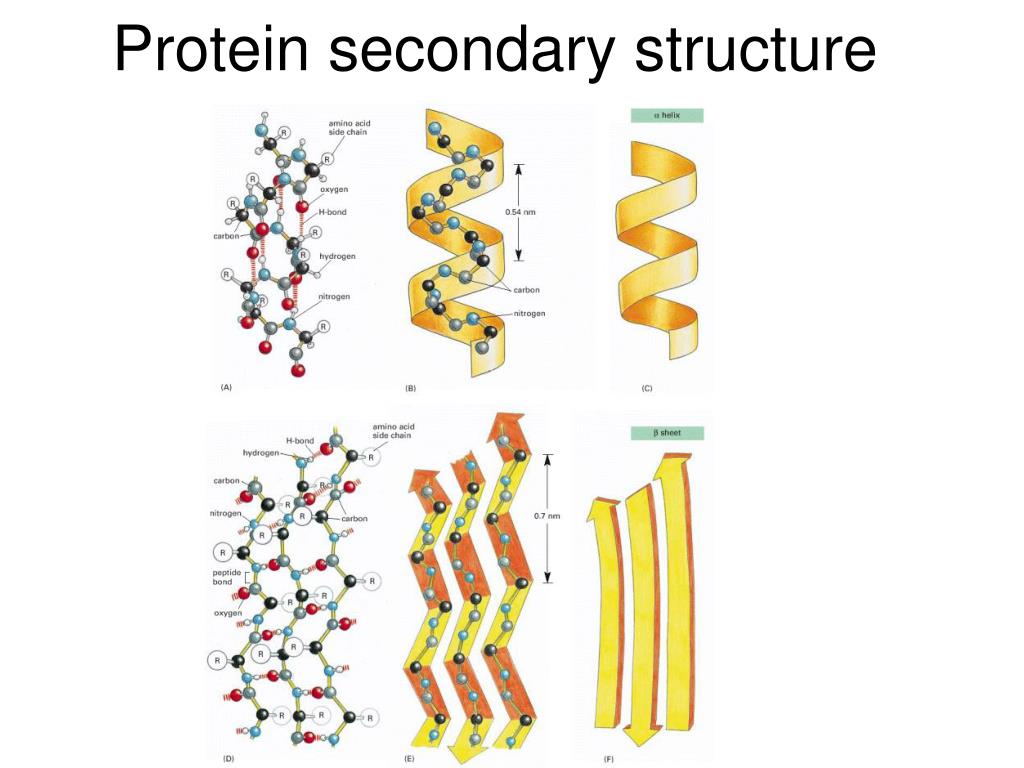
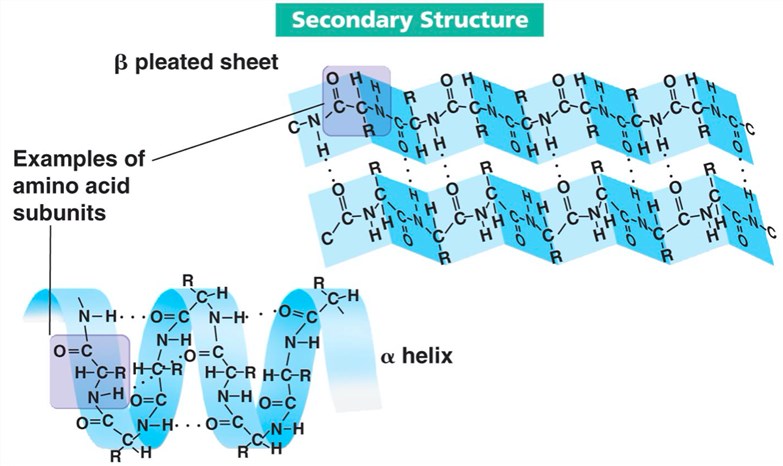
In an alpha-helix, the polypeptide backbone coils tightly around an imaginary axis, forming a helical structure. Hydrogen bonds stabilize this structure by linking the carbonyl oxygen of one amino acid to the amide hydrogen of the amino acid four residues down the chain.
This repeating pattern of hydrogen bonds creates a stable, rod-like structure. All the carbonyl oxygens point in one direction, and all the amide hydrogens point in the opposite direction, reinforcing the dipole moment of the helix.
Side chains of the amino acids extend outward from the helix, allowing them to interact with the surrounding environment. Proline, due to its rigid cyclic structure, and glycine, due to its flexibility, are often found to disrupt alpha-helices.
Beta-Sheets: Strands Aligned in Order
Beta-sheets, in contrast to helices, are formed by strands of the polypeptide backbone lying alongside each other. Hydrogen bonds form between the carbonyl oxygens and amide hydrogens of adjacent strands, linking them together.
These strands can run in the same direction (parallel beta-sheet) or in opposite directions (antiparallel beta-sheet). Antiparallel beta-sheets tend to be more stable because the hydrogen bonds are more linear.
The side chains of amino acids in a beta-sheet alternate, extending above and below the sheet, contributing to the sheet's overall properties. Larger, more bulky side chains often disrupt beta-sheet formation.
Beyond Hydrogen Bonds: Weaker Forces Contribute
While hydrogen bonds are the dominant force maintaining secondary structure, other weaker forces also play a role in influencing the stability and conformation of these elements. These include van der Waals forces and hydrophobic interactions.
Van der Waals forces, resulting from temporary fluctuations in electron distribution, contribute to the overall stability of the packed protein structure. Although individually weak, their cumulative effect can be significant.
Hydrophobic interactions, driven by the tendency of nonpolar amino acid side chains to cluster together away from water, indirectly influence secondary structure. This "hydrophobic effect" can stabilize certain arrangements of alpha-helices and beta-sheets.
The Importance of Secondary Structure
The secondary structure of a protein is not just a static arrangement; it is crucial for its function. The specific arrangement of alpha-helices and beta-sheets determines the protein's overall shape and its ability to interact with other molecules.
Many proteins contain specific structural motifs, such as helix-turn-helix motifs in DNA-binding proteins or beta-barrel structures in membrane proteins, which rely on precisely maintained secondary structures for their function. Any disruption of these structures can compromise protein function.
Misfolding of proteins, often due to disruption of the secondary structure, is implicated in a range of diseases, including Alzheimer's disease, Parkinson's disease, and cystic fibrosis. Understanding how to stabilize and maintain the proper secondary structure of proteins is a major focus of drug development efforts.
Future Directions
Research continues to focus on understanding the intricacies of protein folding and the factors that influence secondary structure formation. Advanced computational methods and experimental techniques, such as X-ray crystallography and cryo-electron microscopy, are providing unprecedented insights into the dynamic nature of protein structure.
By elucidating the mechanisms that maintain secondary structure, scientists hope to develop new strategies for preventing protein misfolding and designing novel therapeutic interventions for diseases linked to protein aggregation and malfunction. This knowledge is crucial for advancing biotechnology, medicine, and our overall understanding of life itself.
In conclusion, hydrogen bonds between the polypeptide backbone are the primary force that maintains the secondary structure of a protein. Understanding the role of these bonds, along with other weaker forces, is crucial for understanding protein function and developing new therapies for diseases related to protein misfolding.