What Makes Each Amino Acid Unique
Life's building blocks, amino acids, aren't created equal. Their unique identities hinge on a single, critical component: the R-group, also known as the side chain.
While all 20 standard amino acids share a common backbone, it's this R-group that dictates their diverse chemical properties and, ultimately, their function within proteins.
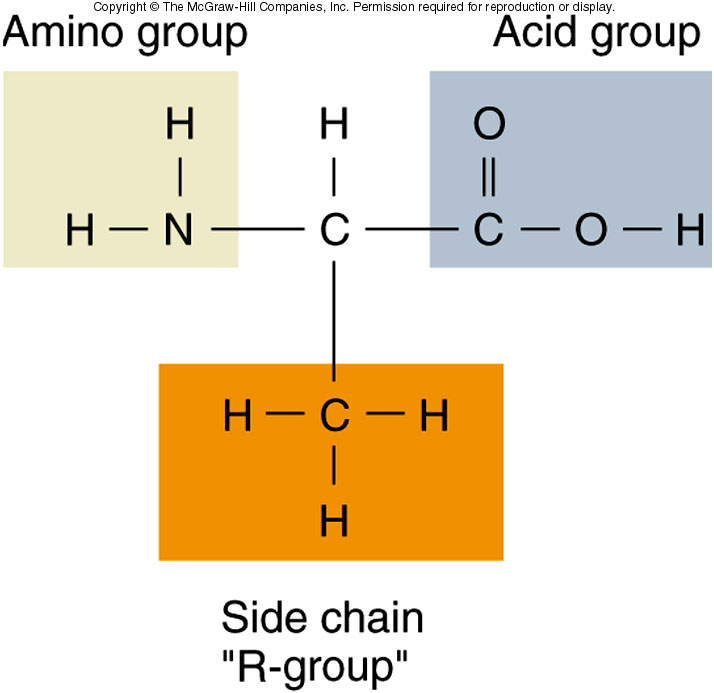
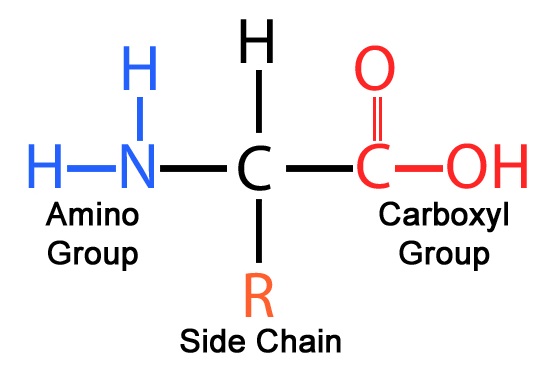
The Common Amino Acid Structure
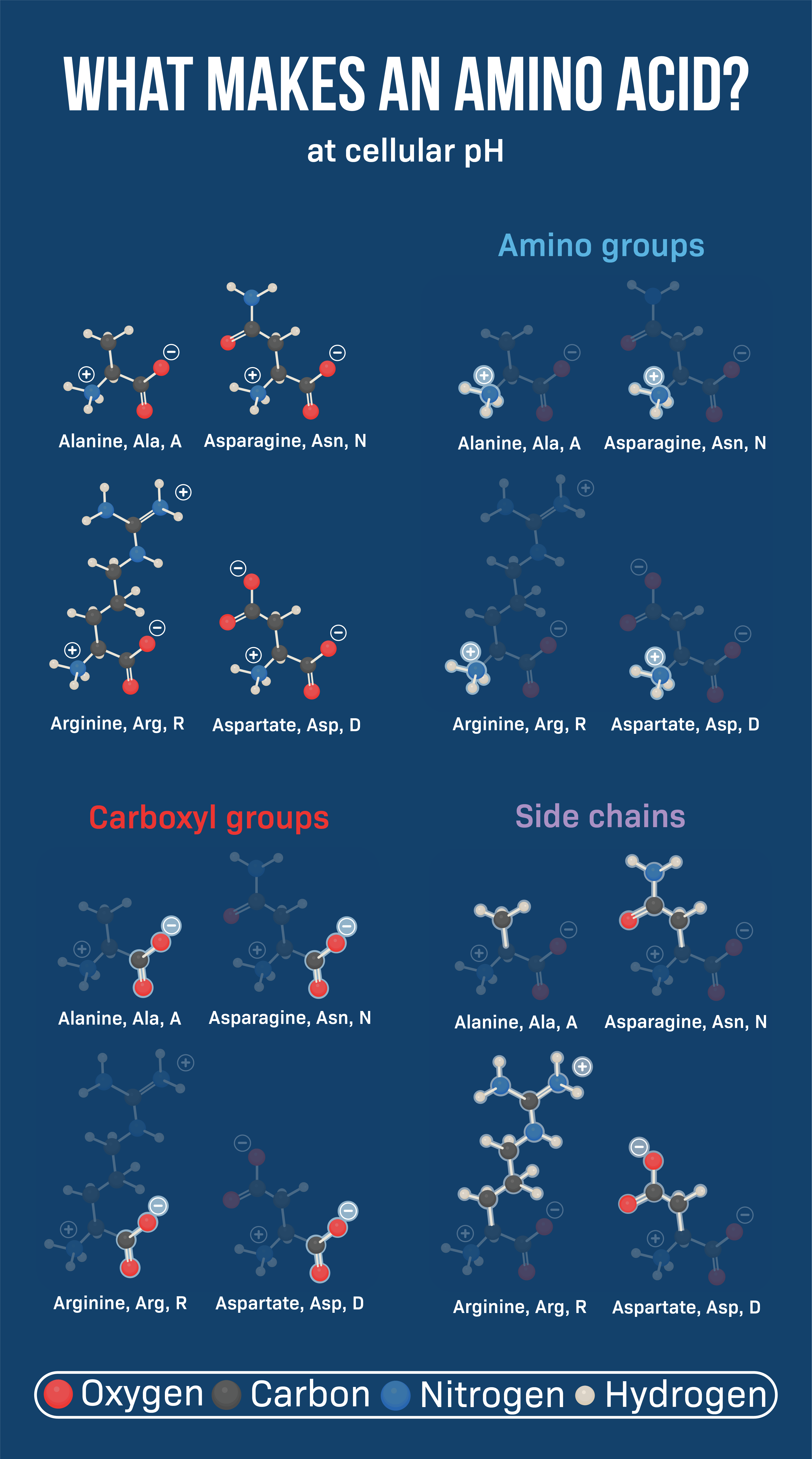
All amino acids share a core structure: a central carbon atom (the alpha carbon) bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom (-H), and the all-important R-group.
This conserved structure allows amino acids to link together via peptide bonds, forming polypeptide chains, the precursors to functional proteins.
Decoding the R-Groups
Each R-group possesses a unique chemical structure, ranging from a single hydrogen atom (in glycine) to complex ring systems.
This structural diversity translates into a wide range of properties, including size, shape, charge, hydrophobicity (water-repelling), and hydrophilicity (water-attracting).
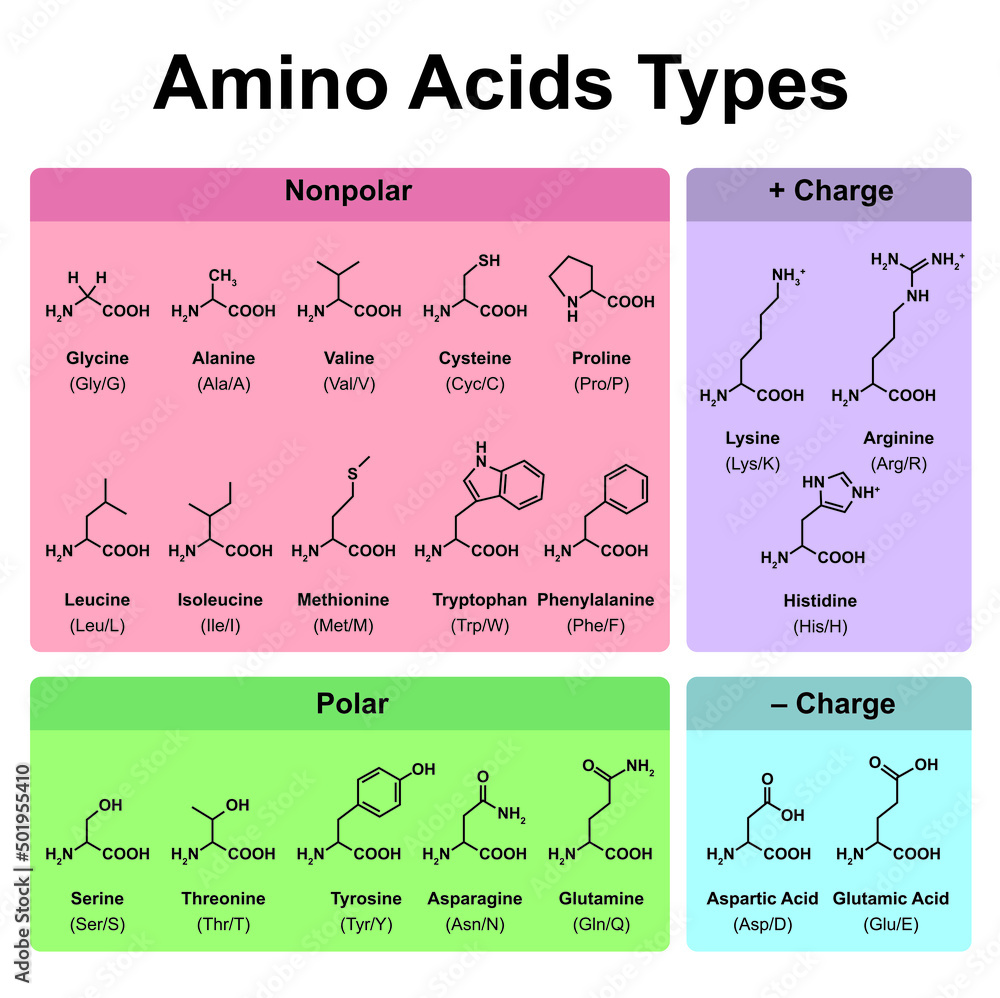
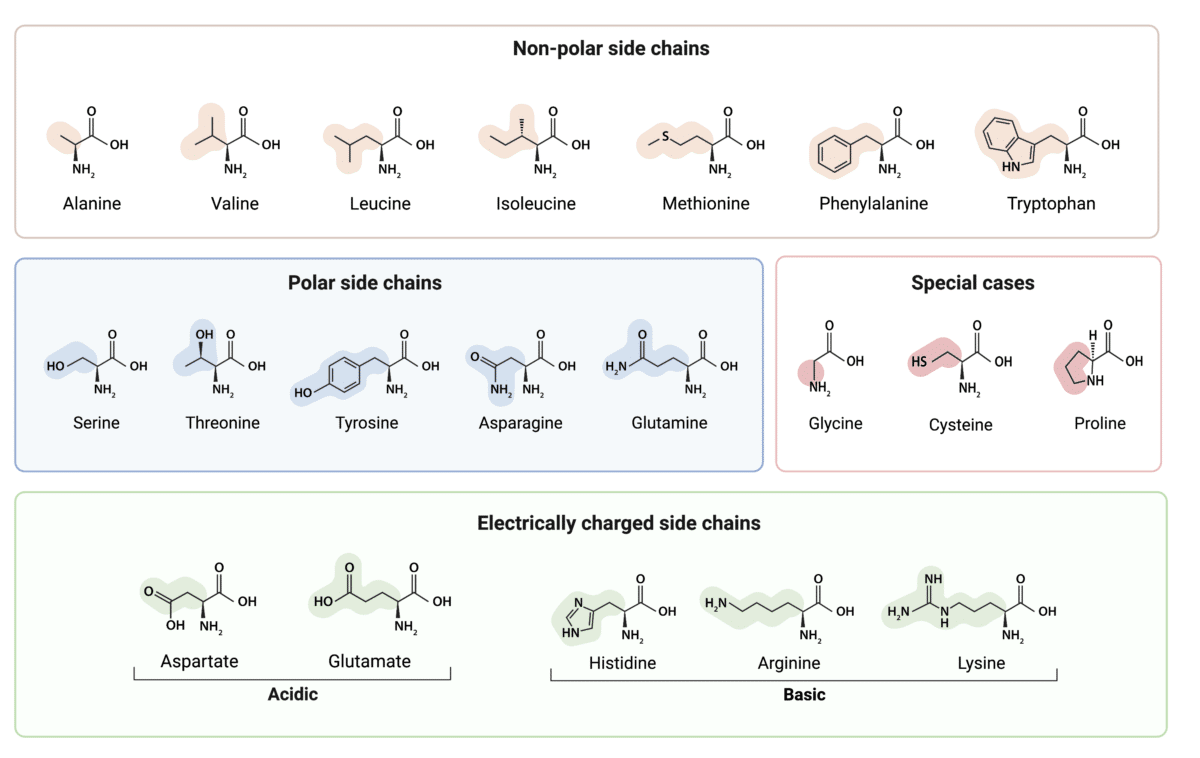
Categorizing by Polarity
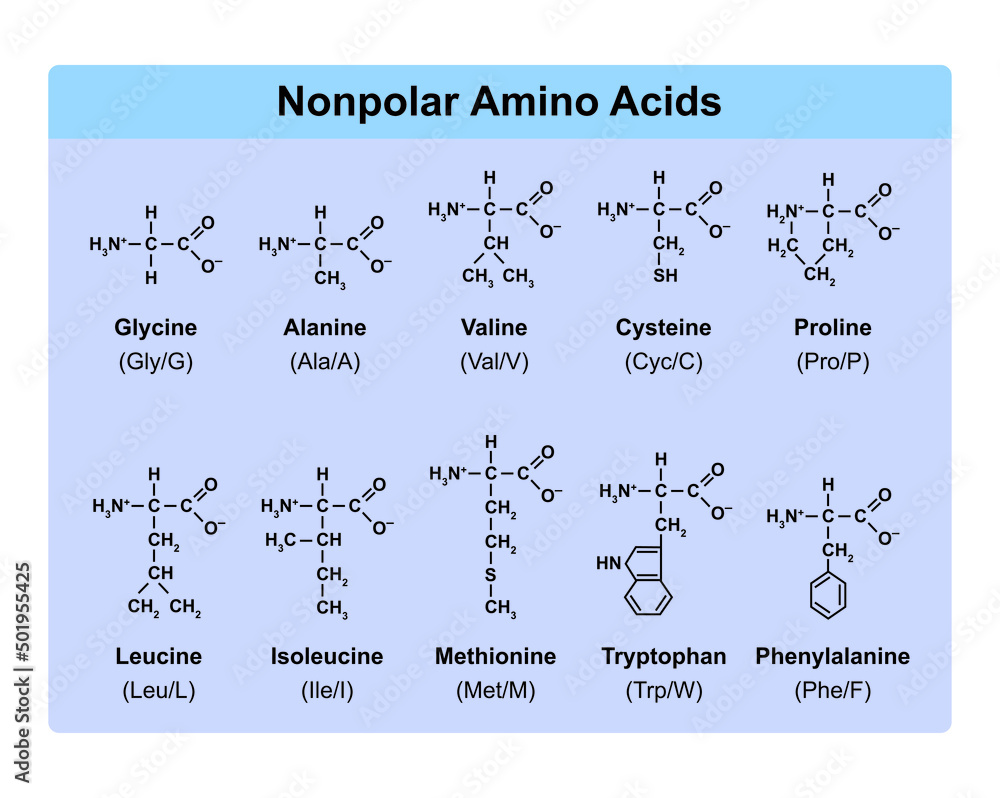
A key categorization of amino acids revolves around their polarity. Polar amino acids have hydrophilic R-groups, while nonpolar amino acids boast hydrophobic R-groups.
Polar amino acids include serine, threonine, cysteine, tyrosine, glutamine, and asparagine. Their R-groups contain atoms like oxygen or nitrogen, allowing them to form hydrogen bonds with water.
Nonpolar amino acids like alanine, valine, leucine, isoleucine, proline, phenylalanine, tryptophan, and methionine, on the other hand, cluster together in the interior of proteins, away from the aqueous environment.
Charged Amino Acids: Acids and Bases
Some amino acids carry a charge at physiological pH (around 7.4). These charged amino acids are either acidic (negatively charged) or basic (positively charged).
Acidic amino acids, aspartic acid and glutamic acid, have carboxyl groups in their R-groups that donate protons, resulting in a negative charge at physiological pH.
Basic amino acids, lysine, arginine, and histidine, have amino groups in their R-groups that accept protons, resulting in a positive charge at physiological pH.
Special Case Amino Acids
Glycine, proline, and cysteine stand out due to their unique structural properties.
Glycine, with its single hydrogen atom R-group, is the smallest amino acid and allows for flexibility in protein structures.
Proline's R-group is cyclic and bonded to the amino group, creating a rigid kink in the polypeptide chain and disrupting alpha-helices and beta-sheets.
Cysteine contains a thiol group (-SH) that can form disulfide bonds with another cysteine residue, stabilizing protein structures.
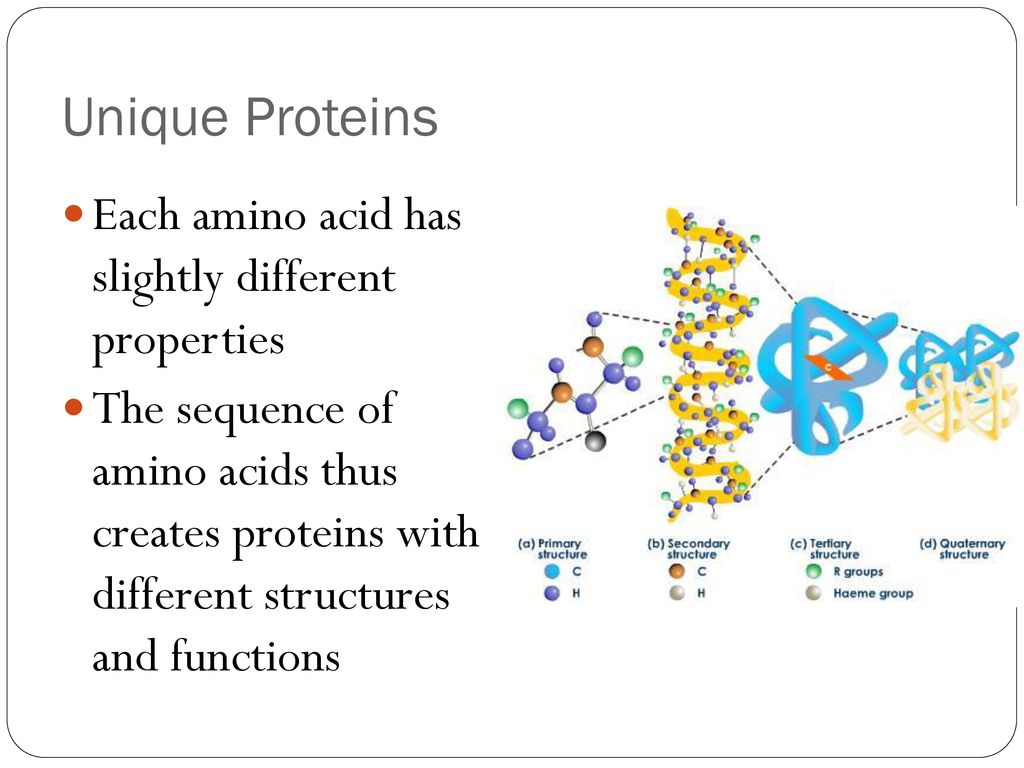
R-Groups Determine Protein Function
The specific arrangement of amino acids, dictated by their R-groups, determines a protein's three-dimensional structure.
This three-dimensional structure, in turn, dictates its function, whether it's catalyzing a biochemical reaction as an enzyme, transporting molecules across cell membranes, or providing structural support.
Mutations that alter the amino acid sequence can have profound consequences on protein function, leading to disease.
Ongoing Research
Researchers continue to investigate the intricate relationship between amino acid structure and protein function using advanced techniques like X-ray crystallography and cryo-electron microscopy.
Understanding this relationship is crucial for developing new drugs that target specific proteins and treating diseases at the molecular level.
Future studies will focus on elucidating the roles of non-standard amino acids and post-translational modifications in expanding protein diversity and function.