What Populations May Have Issues Metabolizing Medications
Medication effectiveness is under threat: Genetic variations and specific health conditions are significantly impacting how different populations metabolize drugs, leading to potential treatment failures and adverse reactions. Understanding these metabolic differences is crucial for optimizing patient care and minimizing harm.
Pharmacogenomics: A Growing Concern
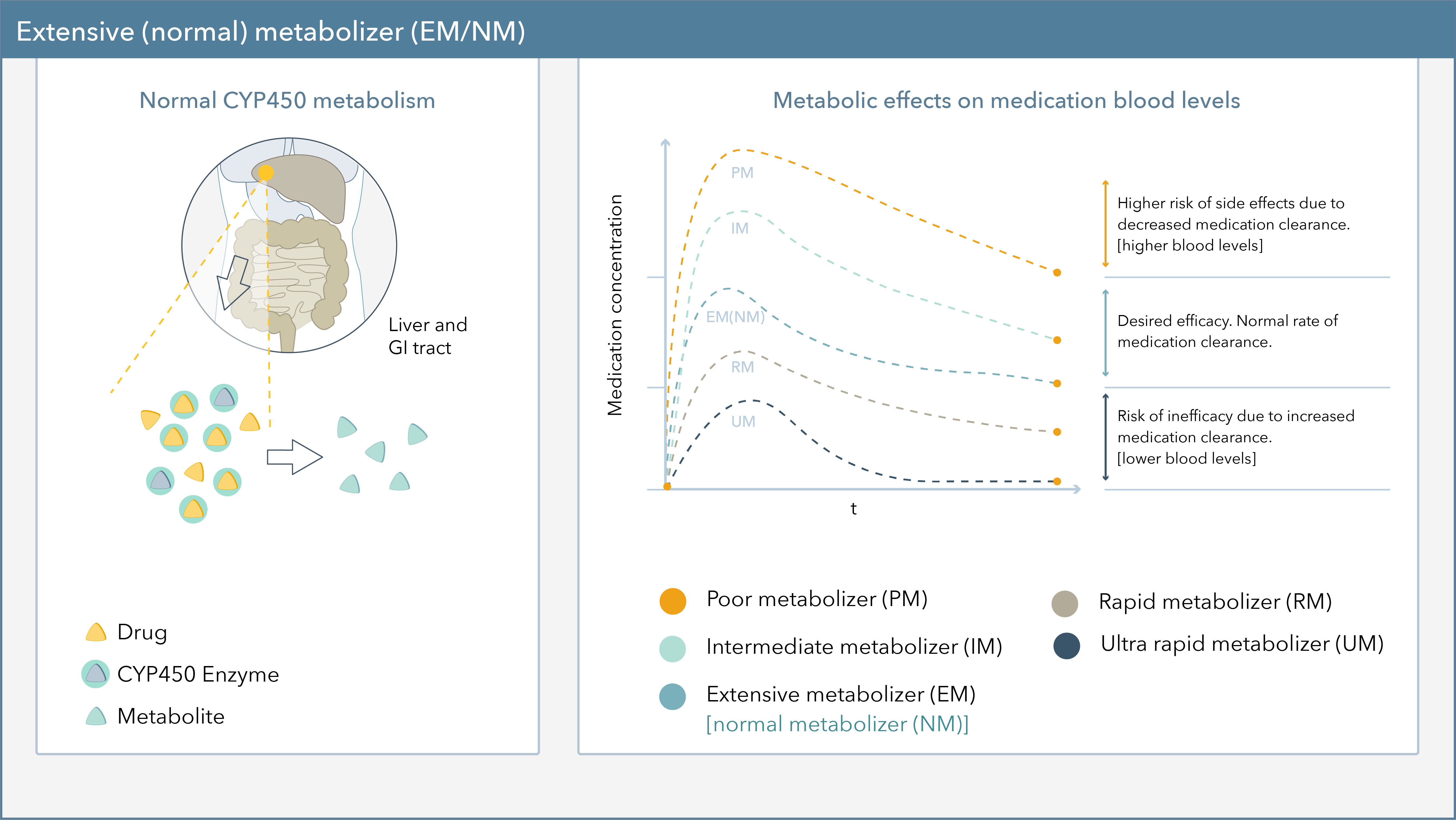
Pharmacogenomics, the study of how genes affect a person's response to drugs, reveals that drug metabolism isn't uniform. Genetic variations, particularly in genes encoding drug-metabolizing enzymes, like cytochrome P450s (CYPs), are key players. These variations can lead to individuals being classified as poor, intermediate, normal, or ultra-rapid metabolizers.
Genetic Variations and Drug Metabolism
CYP2D6 is a highly polymorphic gene responsible for metabolizing approximately 25% of commonly prescribed drugs. Variations in CYP2D6 affect the metabolism of antidepressants, antipsychotics, pain medications (like codeine and tramadol), and beta-blockers. Some populations, like individuals of African descent, may have a higher prevalence of CYP2D6 variants leading to ultra-rapid metabolism, while others, such as those of European descent, are more likely to be poor metabolizers.
Another crucial gene, CYP2C19, influences the metabolism of clopidogrel, a widely used antiplatelet drug. Poor metabolizers of CYP2C19 may not adequately convert clopidogrel to its active form, increasing their risk of stroke or heart attack. This is particularly relevant for East Asian populations, where the prevalence of CYP2C19 loss-of-function alleles can be as high as 70%.
The Impact of Age and Comorbidities
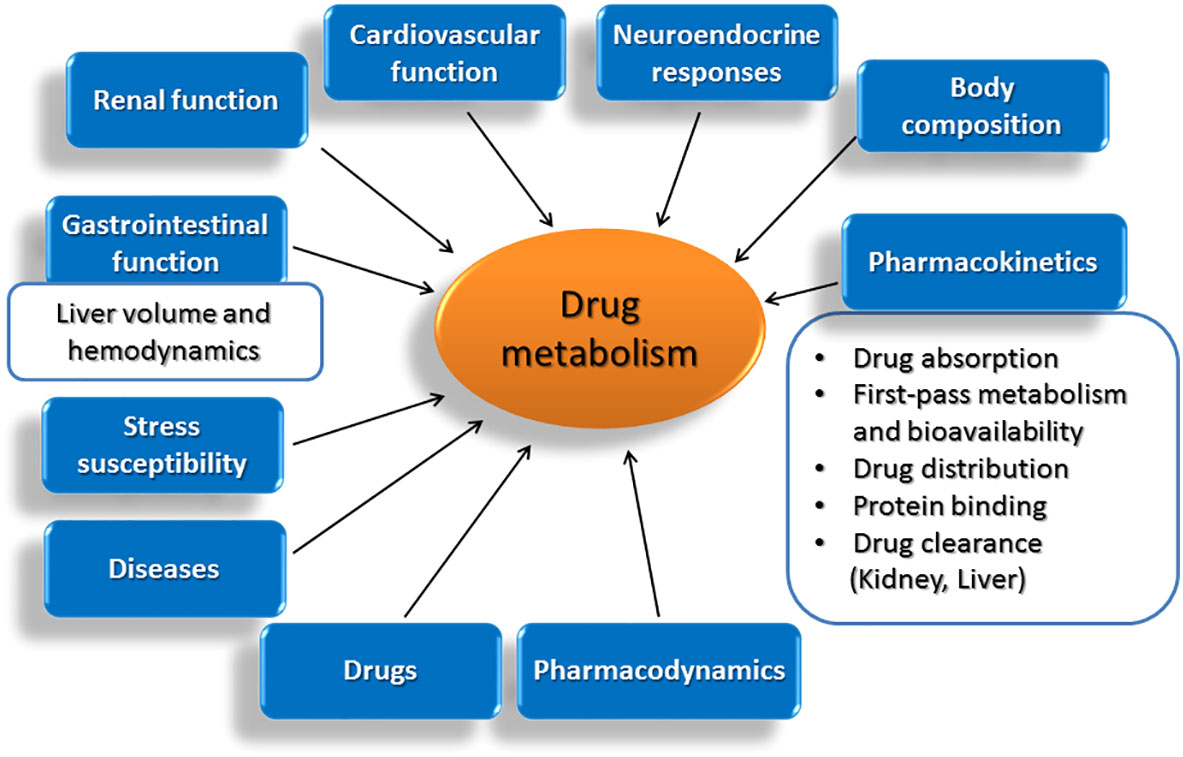
Age significantly impacts drug metabolism. Neonates and infants have immature enzyme systems, making them particularly vulnerable to drug toxicity. Elderly individuals often experience a decline in liver and kidney function, reducing their capacity to eliminate drugs, thus increasing the risk of adverse effects.
Certain diseases dramatically change the way the body processes medications. Liver disease, in particular, impairs the function of drug-metabolizing enzymes. Heart failure can reduce blood flow to the liver and kidneys, hindering drug clearance. Kidney disease directly affects the elimination of many drugs, requiring dosage adjustments.
Ethnic and Racial Disparities
Significant disparities exist across different ethnic and racial groups. For instance, African Americans often have a higher prevalence of genetic variants impacting the metabolism of beta-blockers and antidepressants. This can lead to reduced efficacy or increased side effects with standard dosages.
Asian populations frequently exhibit variations in genes affecting warfarin, an anticoagulant. They often require lower starting doses due to increased sensitivity to the drug, compared to Caucasians. Tailoring dosages based on ethnicity and genetic background can significantly improve treatment outcomes.
The Role of Drug-Drug Interactions
Simultaneous use of multiple medications can significantly alter drug metabolism. One drug can inhibit or induce the activity of drug-metabolizing enzymes, affecting the levels of other drugs in the body. For example, certain antidepressants inhibit CYP2D6, potentially increasing the levels of other drugs metabolized by this enzyme, leading to toxicity.
"Drug-drug interactions are a major concern, particularly in elderly patients who often take multiple medications," warns Dr. Emily Carter, a clinical pharmacologist at University Hospital. "Careful medication reconciliation and dosage adjustments are critical."
Addressing the Challenges: Personalized Medicine
Personalized medicine, using a patient's genetic information to guide treatment decisions, is gaining momentum. Pharmacogenomic testing can identify individuals at risk of adverse drug reactions or treatment failure.
Implementing pharmacogenomic testing into routine clinical practice faces hurdles, including cost, accessibility, and the need for clinician education. However, as the cost of testing decreases and the evidence base grows, personalized medicine is becoming increasingly feasible.
Future Directions and Ongoing Research
Research is ongoing to identify additional genetic variants affecting drug metabolism and to develop more accurate predictive models. Efforts are underway to create clinical decision support tools that integrate pharmacogenomic information to guide prescribing decisions.
The Food and Drug Administration (FDA) is actively updating drug labels to include pharmacogenomic information, assisting healthcare providers in making more informed decisions. Increased awareness and implementation of personalized medicine are essential steps toward improving patient safety and treatment outcomes.