When Did Heplisav B Come Out

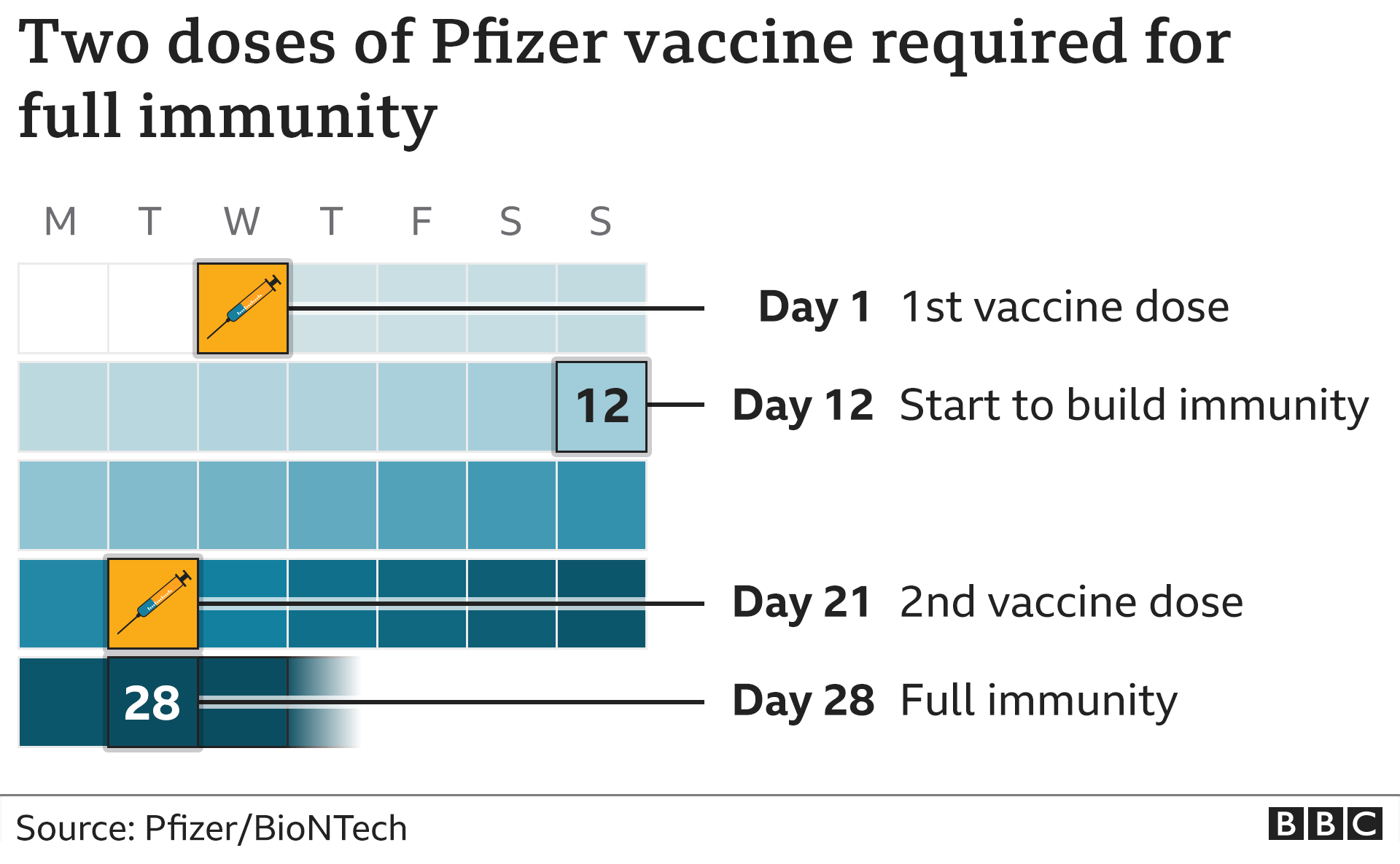
The fight against hepatitis B, a potentially life-threatening liver infection, took a significant turn with the introduction of a novel vaccine: Heplisav B. Unlike its predecessors, Heplisav B offered a shorter, two-dose regimen and boasted improved efficacy, particularly in certain populations. This innovative vaccine promised a faster and more effective shield against a virus that continues to pose a global health challenge.
Heplisav B, manufactured by Dynavax Technologies, received approval from the U.S. Food and Drug Administration (FDA) on November 9, 2017. This marked a pivotal moment in hepatitis B prevention, introducing a new option with a different mechanism of action compared to existing vaccines. Understanding the timeline of its development and introduction is crucial to appreciating its impact on public health.
The Journey to Approval: Development and Clinical Trials
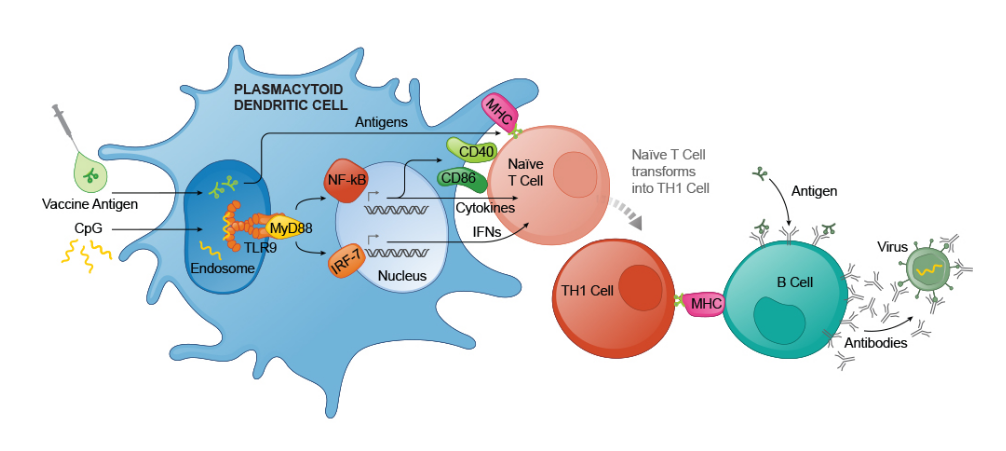
The development of Heplisav B was a multi-year endeavor involving extensive research and clinical trials. Dynavax Technologies focused on creating a vaccine that would overcome some of the limitations of earlier hepatitis B vaccines. Their approach centered around a novel adjuvant, CpG 1018, designed to enhance the immune response.
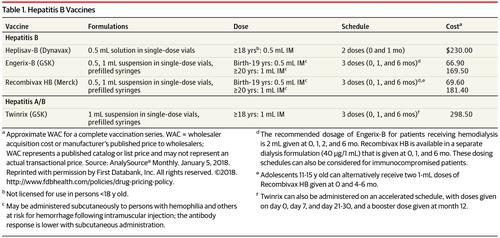
Clinical trials for Heplisav B involved thousands of participants across various age groups and risk categories. These trials rigorously evaluated the vaccine's safety and efficacy. Crucially, studies demonstrated that Heplisav B elicited a higher rate of seroprotection compared to Engerix-B, a commonly used hepatitis B vaccine.
Seroprotection refers to the presence of sufficient levels of antibodies in the blood to protect against infection. The superior seroprotection rates observed with Heplisav B were particularly notable in adults aged 40 and older, a group known to have a weaker response to traditional hepatitis B vaccines.
FDA Approval and Initial Rollout
The FDA's approval of Heplisav B in November 2017 was based on the compelling data from these clinical trials. The approval covered the prevention of hepatitis B infection in adults aged 18 years and older. This was a major step forward for hepatitis B prevention.
Following FDA approval, Dynavax began the process of manufacturing and distributing Heplisav B. The initial rollout involved working with healthcare providers and organizations to ensure the vaccine was available to those who needed it most. This included individuals at high risk of hepatitis B infection, such as healthcare workers and people with certain medical conditions.
The Centers for Disease Control and Prevention (CDC) played a crucial role in disseminating information about Heplisav B and updating vaccination guidelines. The CDC's Advisory Committee on Immunization Practices (ACIP) provided recommendations on the appropriate use of the vaccine.
Adoption and Impact on Vaccination Guidelines
The ACIP initially recommended Heplisav B for adults aged 18 years and older at risk of hepatitis B infection in 2018. This recommendation provided a clear pathway for healthcare providers to incorporate the vaccine into their practice.
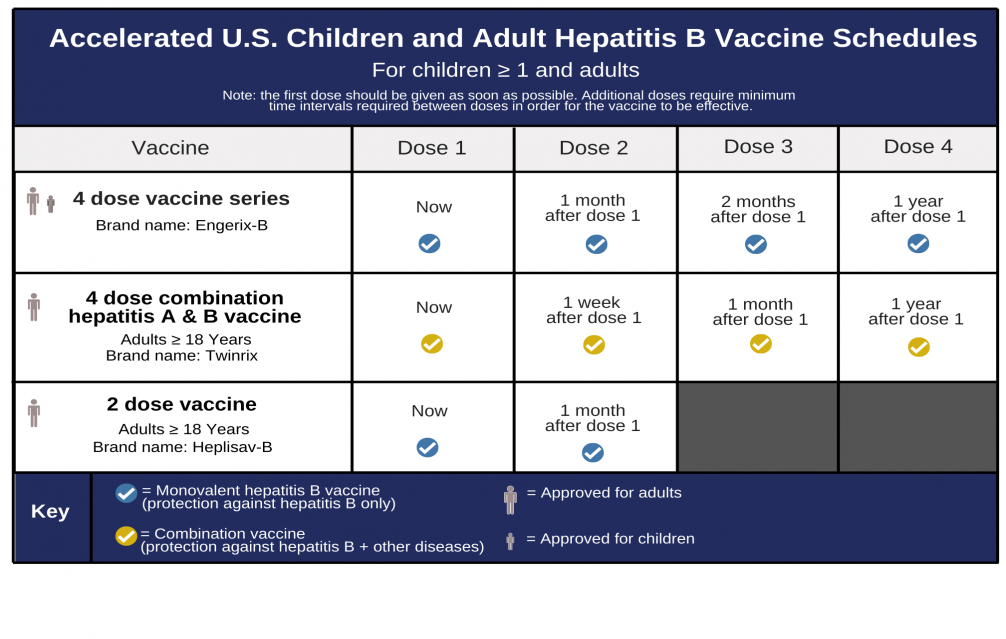
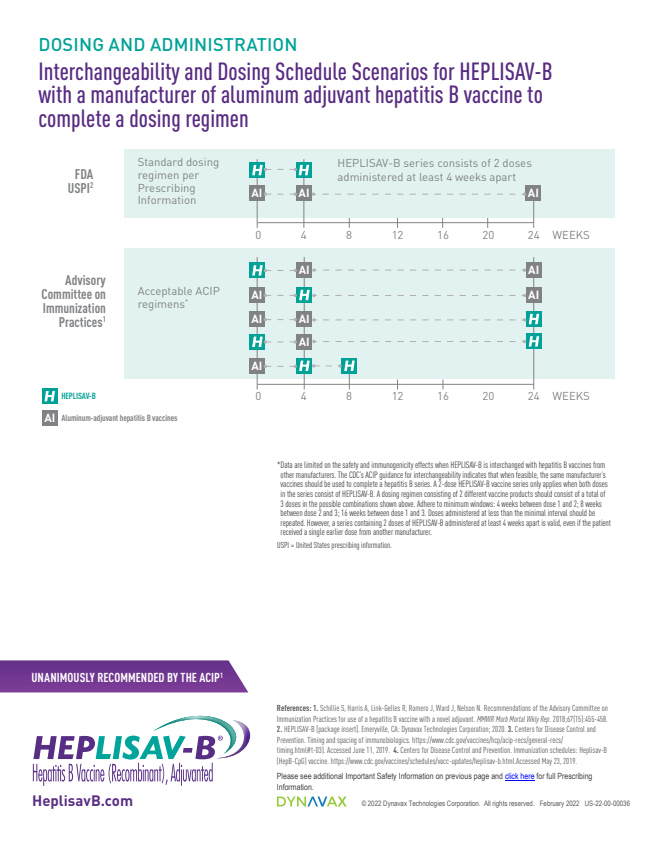
Over time, the ACIP's recommendations evolved, reflecting the growing body of evidence supporting Heplisav B's effectiveness and safety. In 2022, the ACIP updated its recommendations to state that all adults aged 19-59 years should receive hepatitis B vaccination, and that adults 60 years and older should also be vaccinated if they have risk factors or request vaccination. This broadened the scope of vaccination efforts.
The updated recommendations further emphasized the use of either a two-dose series of Heplisav B or a three-dose series of another hepatitis B vaccine. This provided healthcare providers with flexibility in choosing the most appropriate vaccine for their patients.
Heplisav B: Advantages and Considerations
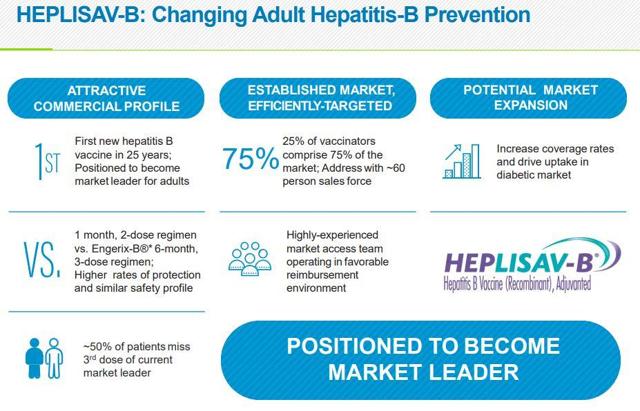
Heplisav B offers several advantages over traditional hepatitis B vaccines. The two-dose regimen is more convenient for patients, leading to improved adherence. The enhanced immune response, particularly in older adults and individuals with certain medical conditions, is also a significant benefit.
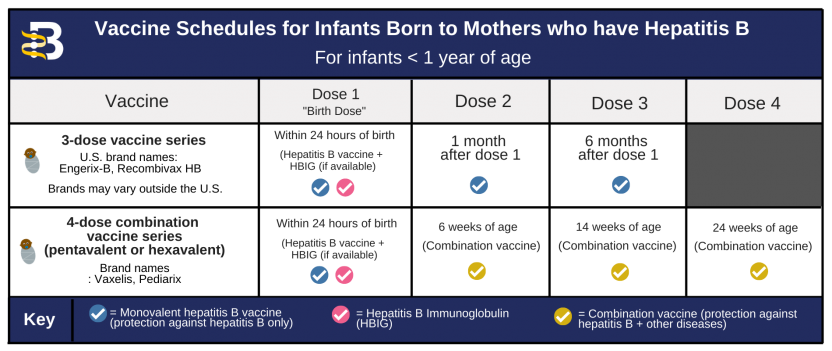
However, it's important to consider potential drawbacks. Heplisav B is currently only approved for adults, not for children. The cost of the vaccine may also be a barrier for some individuals and healthcare systems.
Like all vaccines, Heplisav B can cause side effects. The most common side effects are pain, redness, and swelling at the injection site, as well as fatigue and headache. Serious adverse events are rare.
The Future of Hepatitis B Prevention
Heplisav B represents a significant advancement in the fight against hepatitis B. Its introduction has contributed to increased vaccination rates and improved protection against this potentially deadly virus. Its arrival spurred innovation.
Ongoing research continues to explore new strategies for hepatitis B prevention and treatment. This includes the development of new vaccines and antiviral therapies. Eradicating hepatitis B remains a global health priority.
The story of Heplisav B is a testament to the power of scientific innovation in addressing public health challenges. From its initial development to its widespread adoption, this vaccine has played a vital role in protecting individuals from the devastating effects of hepatitis B. It is a critical tool that continues to evolve.