When Will Btk Inhibitors Be Approved For Ms

Imagine a world where the frustrating uncertainty of multiple sclerosis (MS) is met with a new wave of targeted therapies, offering hope for a brighter, more predictable future. Sunlight streams through a clinic window, illuminating a brochure promising advancements in MS treatment. The air is filled with quiet anticipation as patients and their families discuss the potential of BTK inhibitors, a new class of drugs that might finally shift the paradigm in managing this complex condition.
While disease-modifying therapies (DMTs) for MS have significantly improved over the years, BTK inhibitors represent a potentially game-changing approach. The pivotal question on the minds of many is: when will these innovative drugs be approved for MS, bringing much-needed relief and slowing disease progression for those living with this challenging condition?
Understanding MS and the Current Treatment Landscape
Multiple sclerosis is a chronic autoimmune disease affecting the central nervous system, including the brain and spinal cord. The immune system mistakenly attacks the myelin sheath, the protective covering around nerve fibers, leading to inflammation and damage.
This damage disrupts communication between the brain and the body, resulting in a wide range of symptoms such as fatigue, numbness, muscle weakness, and vision problems. The course of MS is highly variable, with some individuals experiencing mild symptoms while others face significant disability.
Existing DMTs primarily target the adaptive immune system, which includes T cells and B cells. These therapies aim to reduce the frequency and severity of relapses and slow the overall progression of the disease.
While current treatments have proven beneficial, they are not without limitations, including potential side effects and varying degrees of efficacy. Some individuals with MS continue to experience disease progression despite being on these treatments, highlighting the need for more effective and targeted therapies.
The Promise of BTK Inhibitors
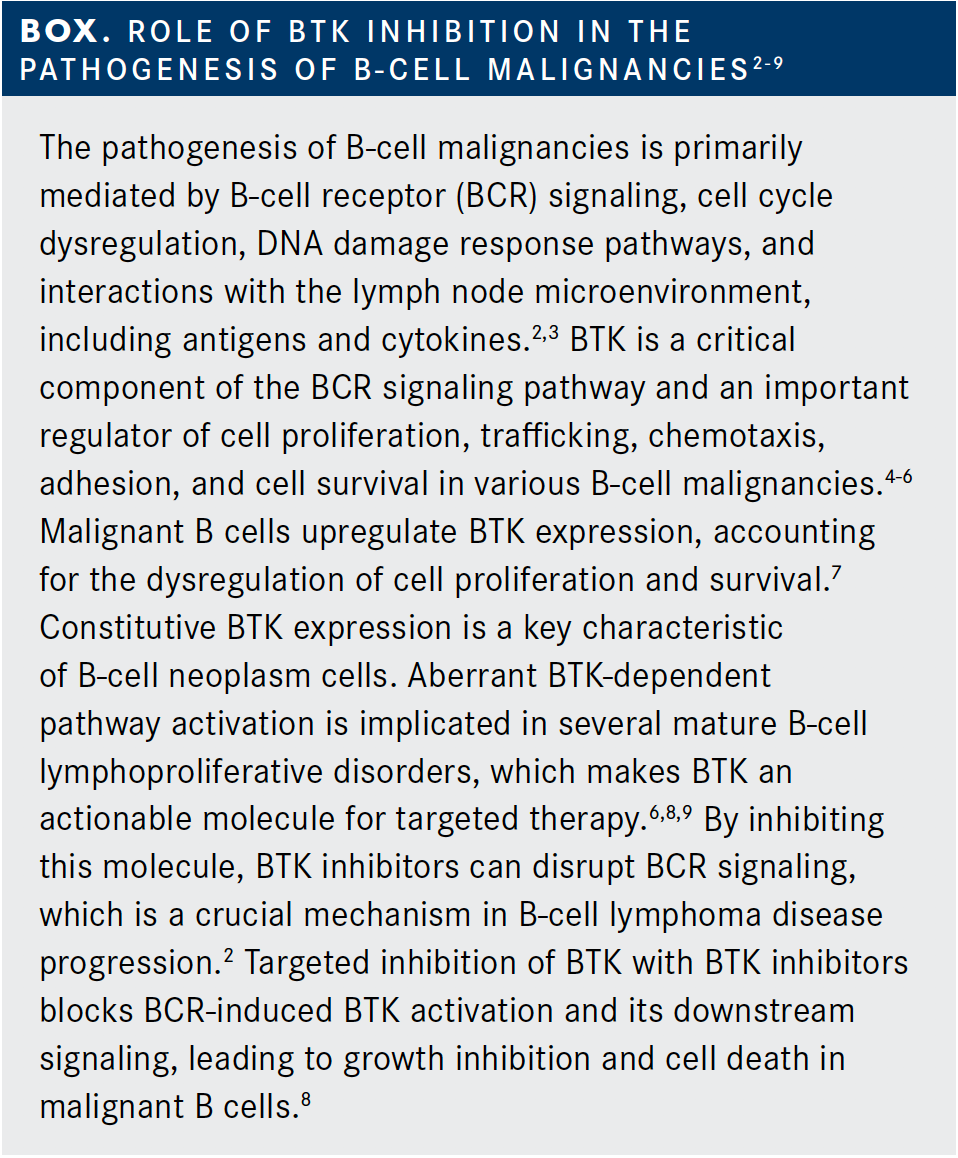
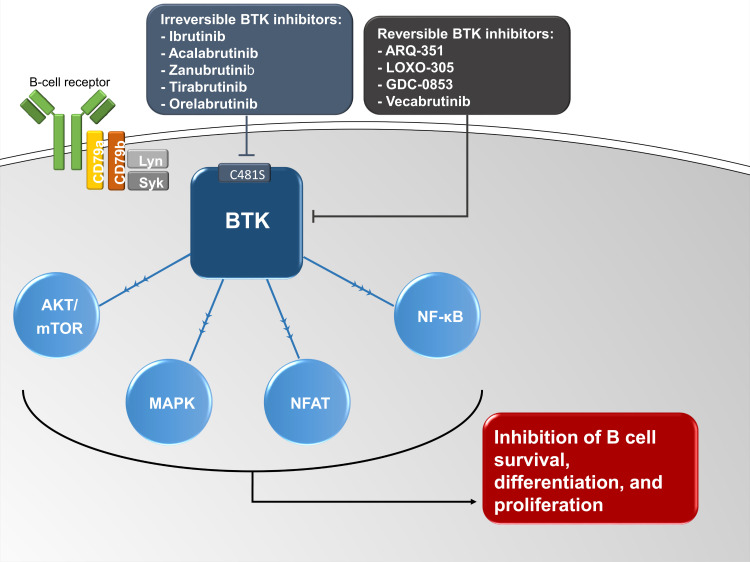
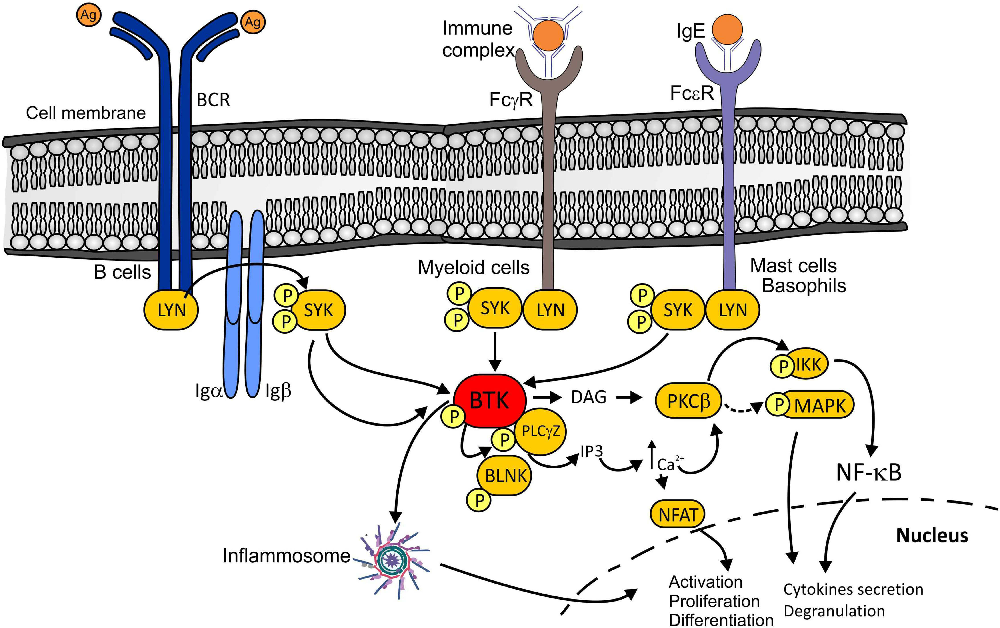
BTK inhibitors work by targeting Bruton's tyrosine kinase (BTK), an enzyme crucial for the function of B cells and myeloid cells, including microglia. Microglia, the brain's resident immune cells, play a significant role in the inflammation and neurodegeneration associated with MS.
By inhibiting BTK, these drugs aim to dampen the activity of both B cells in the periphery and microglia within the central nervous system. This dual action could potentially offer a more comprehensive approach to managing MS by addressing both inflammation and neurodegeneration.
Unlike many existing MS treatments that primarily target the adaptive immune system, BTK inhibitors have the potential to modulate the innate immune system's response in the brain. This could lead to a more significant impact on disease progression, particularly in progressive forms of MS where inflammation within the brain plays a critical role.
Clinical Trials and Emerging Data
Several BTK inhibitors are currently under investigation in clinical trials for various forms of MS, including relapsing-remitting MS (RRMS) and progressive MS. These trials are evaluating the safety and efficacy of these drugs in reducing relapse rates, slowing disability progression, and improving other clinical outcomes.
Early results from some clinical trials have been promising, demonstrating a reduction in new brain lesions and a potential impact on disability progression. However, it's important to note that these are still early days, and larger, longer-term studies are needed to confirm these findings and fully assess the safety profile of BTK inhibitors.
Researchers are particularly interested in understanding the long-term effects of BTK inhibition on the immune system and the potential for any unforeseen side effects. These ongoing studies will provide valuable insights into the role of BTK inhibitors in the treatment of MS and help guide future clinical practice.
Regulatory Pathways and Approval Timelines
The approval of new drugs like BTK inhibitors is a rigorous process overseen by regulatory agencies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe. These agencies carefully evaluate the data from clinical trials to determine whether the benefits of a drug outweigh its risks.
The timeline for approval can vary depending on several factors, including the size and complexity of the clinical trial data, the priority assigned to the application, and any potential safety concerns identified during the review process. Typically, the process involves submitting a comprehensive application with detailed information on the drug's efficacy, safety, manufacturing, and quality control.
While it is difficult to predict exact approval dates, industry analysts and experts closely monitor the progress of clinical trials and regulatory submissions to estimate potential timelines. Based on current data and ongoing trials, some analysts anticipate that the first BTK inhibitors could potentially receive regulatory approval for MS within the next one to two years, but this remains subject to change based on the regulatory review process.
Delays can occur if the regulatory agencies require additional data or have concerns about the safety or efficacy of the drug. It's crucial for patients to stay informed through reliable sources such as their neurologists, MS organizations, and official announcements from the pharmaceutical companies developing these drugs.
Impact on the MS Community
The potential approval of BTK inhibitors for MS has generated considerable excitement within the MS community. Many individuals living with MS and their families are eager to have access to new treatment options that could offer better disease control and improve their quality of life.
The possibility of a therapy that targets both the adaptive and innate immune systems is particularly appealing, as it could potentially address some of the underlying mechanisms driving disease progression in MS. This could translate into a more significant impact on disability, fatigue, and other debilitating symptoms.
However, it's also important to manage expectations and recognize that BTK inhibitors are not a cure for MS. They are likely to be another tool in the toolbox for managing the disease, and their effectiveness may vary from person to person.
Open communication between patients and their healthcare providers is essential to determine whether BTK inhibitors are an appropriate treatment option and to discuss potential risks and benefits. The integration of BTK inhibitors into the existing treatment paradigm will likely require careful consideration of individual patient characteristics and disease activity.
Looking Ahead
The development of BTK inhibitors represents a significant advancement in the field of MS research. These drugs have the potential to offer a new and more targeted approach to managing the disease, addressing both inflammation and neurodegeneration.
As clinical trials continue to progress and regulatory reviews unfold, the MS community remains hopeful that BTK inhibitors will soon become a valuable addition to the available treatment options. The approval of these drugs could mark a new era in MS care, offering individuals living with this condition a brighter outlook and improved quality of life.
While the exact timeline for approval remains uncertain, the dedication of researchers, clinicians, and pharmaceutical companies to advancing MS treatment is unwavering. Their collective efforts are paving the way for a future where MS is managed more effectively, and individuals can live fuller, more active lives.
The journey toward better treatments for MS is a continuous one, filled with challenges and breakthroughs. The story of BTK inhibitors is a testament to the power of scientific innovation and the unwavering hope of the MS community for a better tomorrow.