When Will Humacyte Inc Lated Drugs Be Released

Humacyte Inc., a biotechnology company pioneering engineered human tissues, has generated significant interest within the medical community, particularly regarding the anticipated release dates of its late-stage drug candidates. The company's focus on developing off-the-shelf, implantable human tissues has the potential to revolutionize treatment options for a variety of vascular and other medical conditions. This article examines the current status of Humacyte's pipeline and explores the projected timelines for potential market availability of its key products.
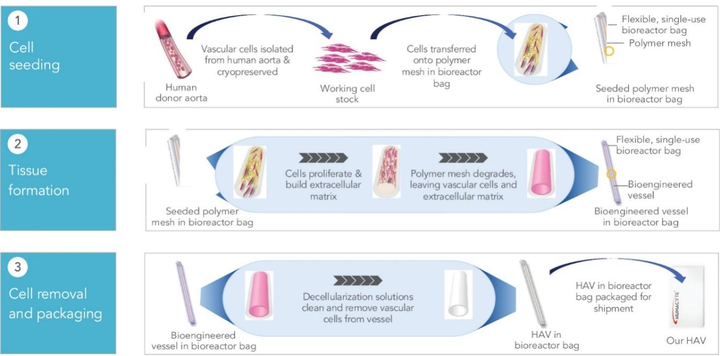
At the core of Humacyte's innovation is its Human Acellular Vessels (HAVs). These bioengineered blood vessels are designed to be readily available for implantation, eliminating the need for patient-specific tissue harvesting and reducing the risks associated with traditional grafts.
Humacyte's most advanced program focuses on the HAV for vascular trauma repair. This is a critical need, especially in emergency situations where time is of the essence.
Humacyte's Pipeline: Key Products and Timelines
Currently, Humacyte’s pipeline features several promising drug candidates in various stages of development. Here's a breakdown of the key products and their anticipated timelines:
HAV for Vascular Trauma
The HAV for vascular trauma has garnered the most attention due to its potential to address a significant unmet need in reconstructive surgery. The company submitted a Biologics License Application (BLA) to the Food and Drug Administration (FDA) in April 2023.
The submission was based on positive results from the VASCULAR trial, a Phase III study demonstrating the safety and efficacy of the HAV in repairing vascular injuries. However, in August 2023, Humacyte received a Complete Response Letter (CRL) from the FDA regarding the BLA.
The CRL indicated that the FDA required additional information to support the application. Humacyte has been working to address the FDA’s concerns.
Humacyte expects to resubmit the BLA for the HAV for vascular trauma in the second half of 2024, with a potential FDA decision in the first half of 2025. This timeline is subject to change based on the FDA's review process.
HAV for Dialysis Access
Another significant application of Humacyte's HAV technology is for creating vascular access in patients undergoing hemodialysis. Many patients with end-stage renal disease require a reliable vascular access point for dialysis.
Humacyte is also developing the HAV for arteriovenous (AV) access in hemodialysis. Clinical trials are ongoing to evaluate the safety and efficacy of the HAV in this setting.
The HAV for dialysis access is currently in Phase III clinical trials. Humacyte anticipates data readout from these trials in late 2024 or early 2025.
Following the data readout, Humacyte expects to submit a BLA to the FDA. If approved, the HAV for dialysis access could offer a more durable and less complication-prone alternative to existing vascular access options.
Humacyl: HAV for Peripheral Arterial Disease
Humacyl, also known as the HAV for peripheral arterial disease (PAD), represents another crucial area of focus for Humacyte. PAD affects millions worldwide and can lead to severe complications.
Humacyte has been conducting clinical trials to evaluate the safety and efficacy of Humacyl in treating PAD. Results have been promising, suggesting that Humacyl may improve blood flow.
Humacyte has not yet announced a definitive timeline for BLA submission for Humacyl. More information on the anticipated regulatory pathway will likely be shared following the completion of ongoing clinical trials and data analysis.
Potential Impact and Significance
The potential impact of Humacyte’s technologies on the medical landscape is significant. The availability of off-the-shelf, bioengineered human tissues could transform the treatment of vascular diseases.
This would address critical unmet needs in reconstructive surgery, dialysis access, and peripheral arterial disease. Moreover, the HAV technology could reduce the reliance on autologous grafts, minimizing patient morbidity and recovery times.
The successful commercialization of Humacyte's HAVs could also pave the way for the development of engineered tissues for other organ systems. This could potentially revolutionize the treatment of various diseases and injuries.
Challenges and Considerations
Despite the promising potential, Humacyte faces several challenges. Securing regulatory approval from the FDA is a complex and rigorous process.
Manufacturing bioengineered tissues at scale is another significant hurdle. Humacyte needs to demonstrate its ability to produce high-quality HAVs consistently to meet future demand.
Reimbursement is crucial for the widespread adoption of Humacyte's technologies. If approved, Humacyte will need to work with payers to ensure adequate coverage and reimbursement for its HAVs.
Conclusion
Humacyte Inc. is at a pivotal juncture as it progresses towards potential commercialization of its HAV technology. While the exact release dates of its late-stage drug candidates remain subject to regulatory review and other factors, the company's progress is being closely watched by the medical community.
The potential impact of Humacyte's HAVs on the treatment of vascular diseases is considerable. The anticipated timelines for BLA resubmission and data readouts provide a roadmap for the coming years.
The next 12-18 months will be critical for Humacyte as it navigates the regulatory pathway and prepares for potential commercial launch. The company's success could usher in a new era of regenerative medicine and transform the treatment of a range of debilitating conditions.