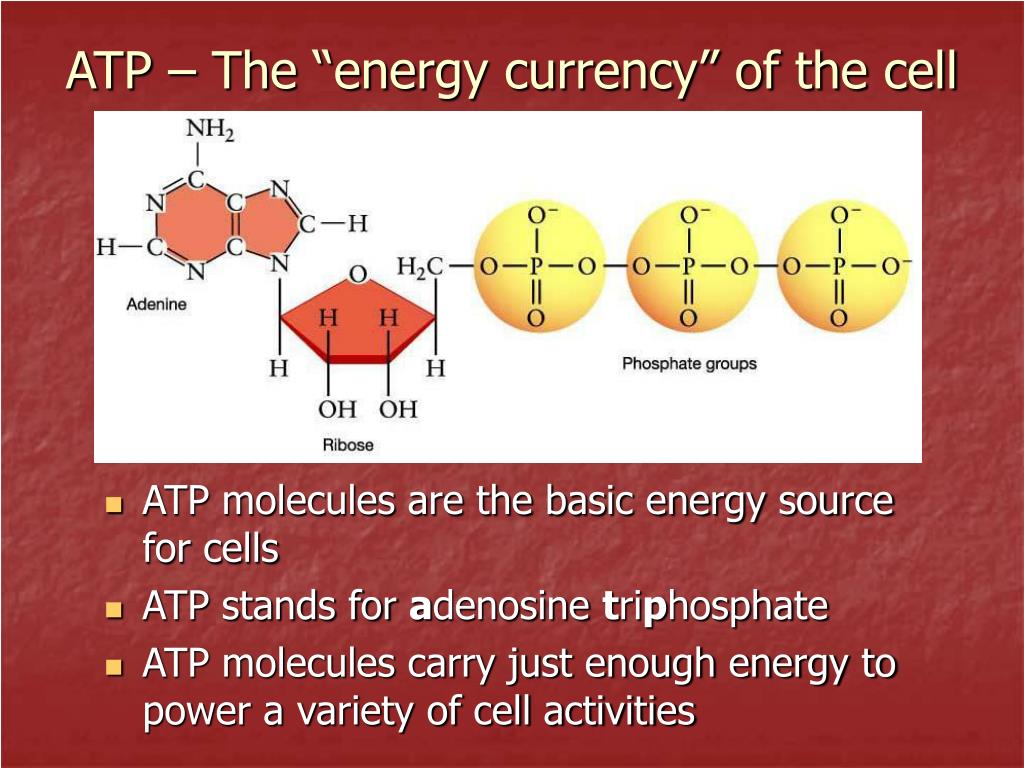
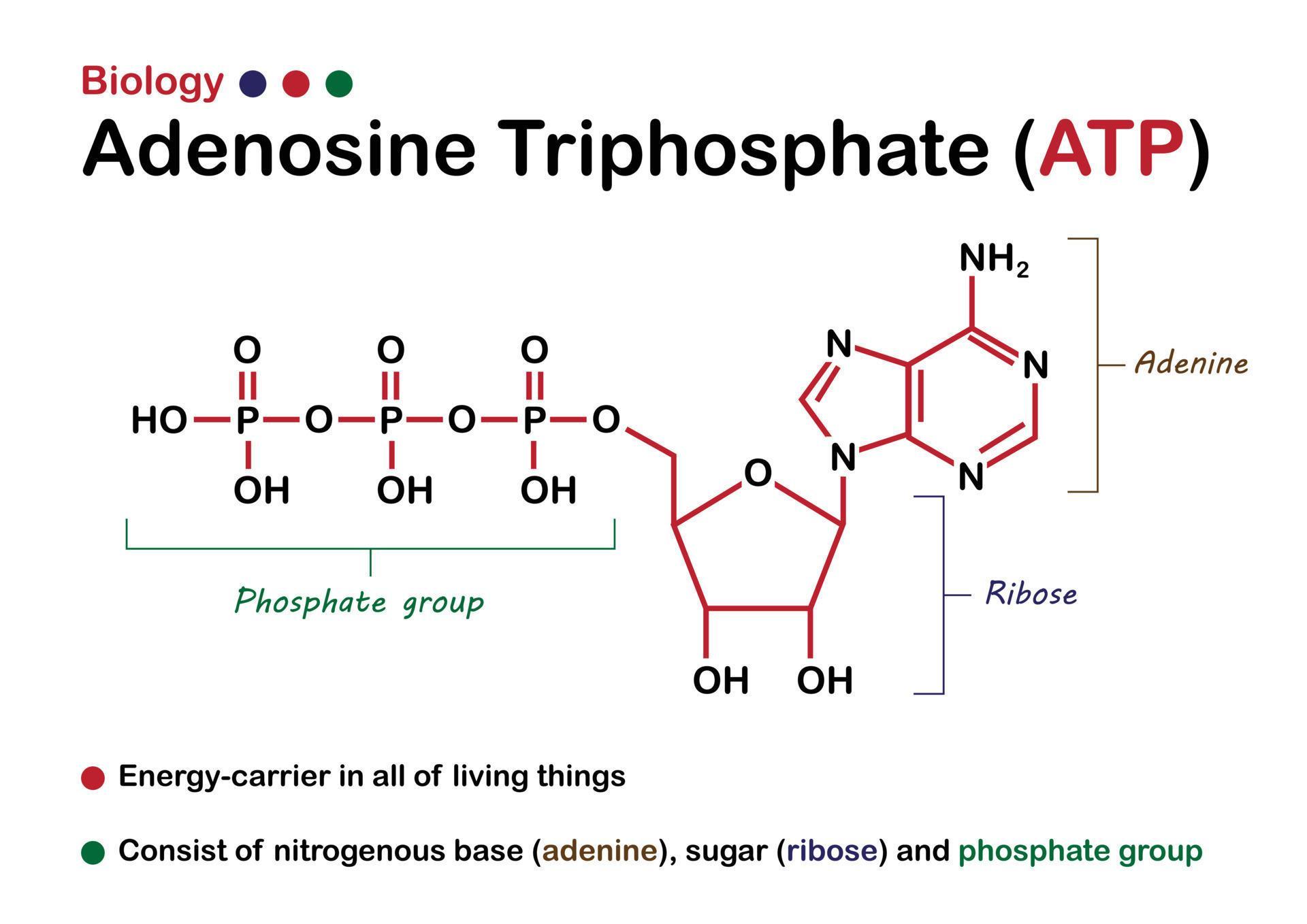
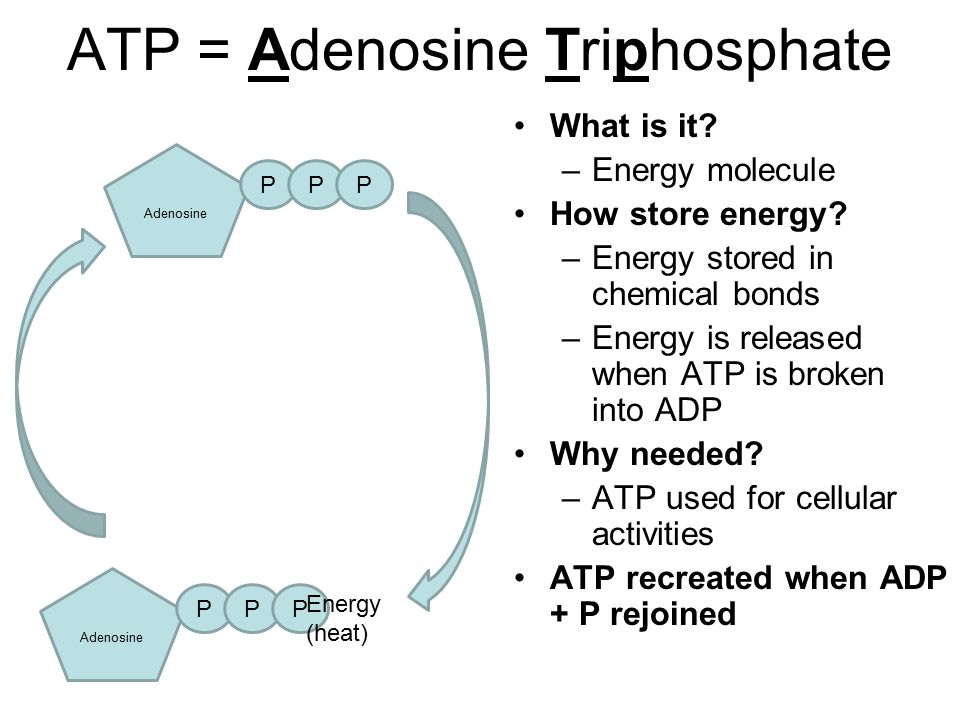
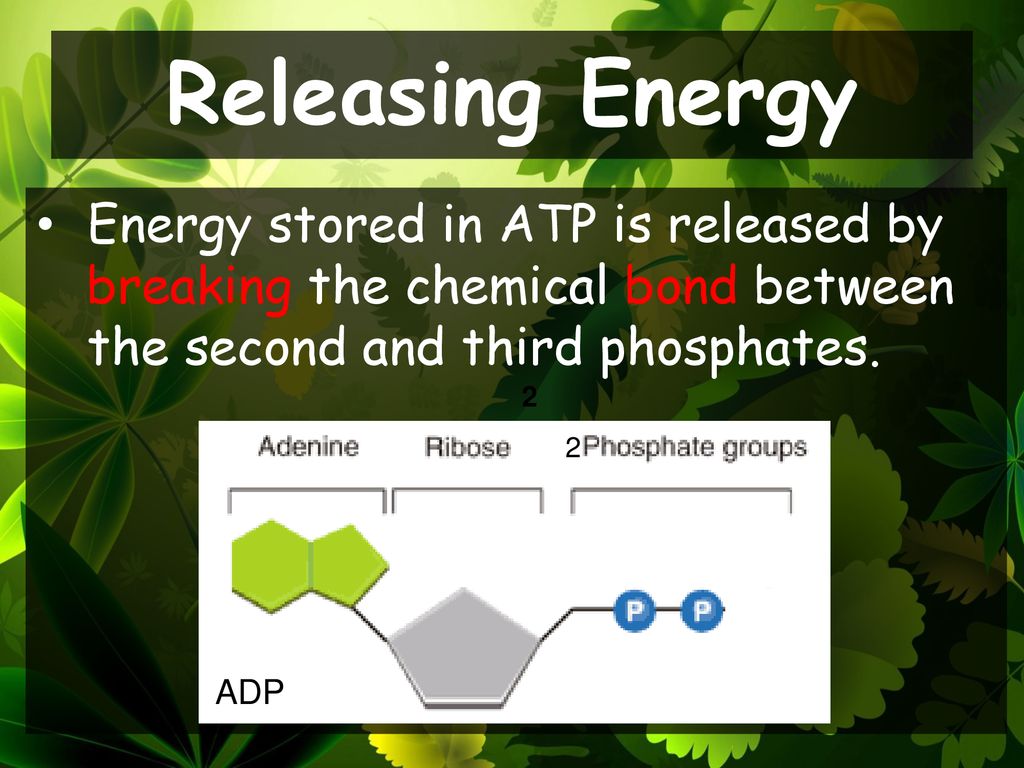
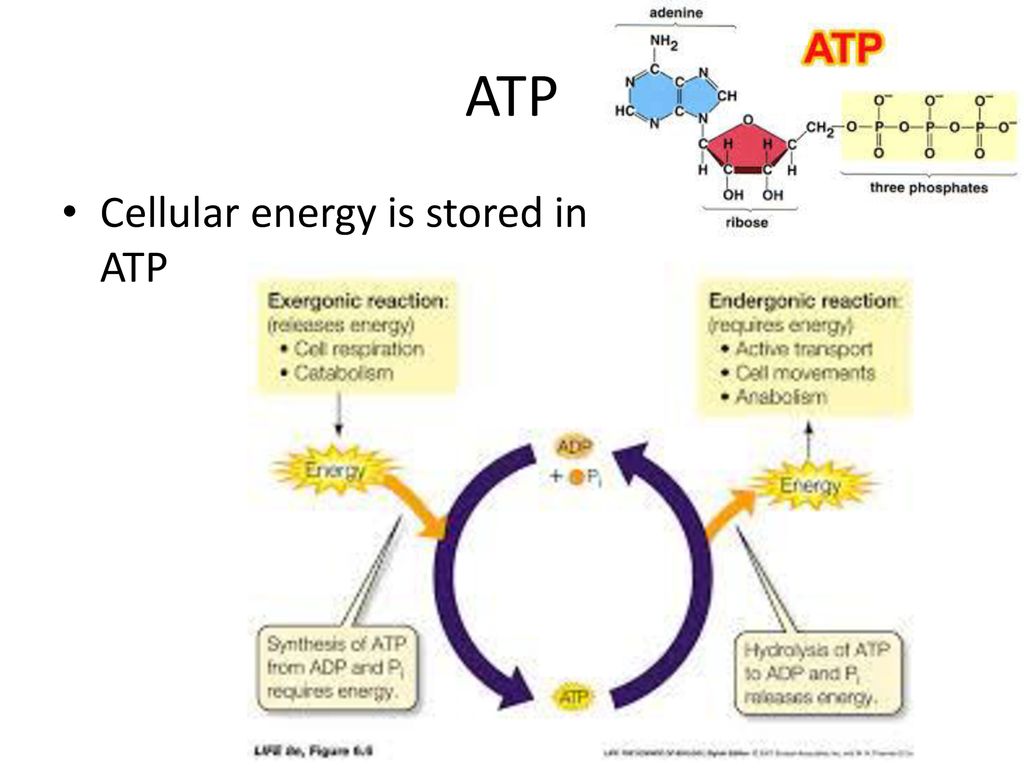
Where Is The Energy Stored In Atp
Scientists have pinpointed the precise location of energy storage within ATP molecules, resolving a decades-long debate with implications for drug development and our fundamental understanding of cellular processes. This breakthrough clarifies how cells power their activities, offering new avenues for manipulating energy transfer at the molecular level.
The mystery surrounding ATP's energy storage has finally been cracked: the energy isn't stored in the bonds themselves, but in the energetic state caused by the repulsion of negatively charged phosphate groups. This understanding revolutionizes our comprehension of cellular energy dynamics and could pave the way for groundbreaking advancements in medicine and biotechnology.
The Core Discovery: Repulsion is Key
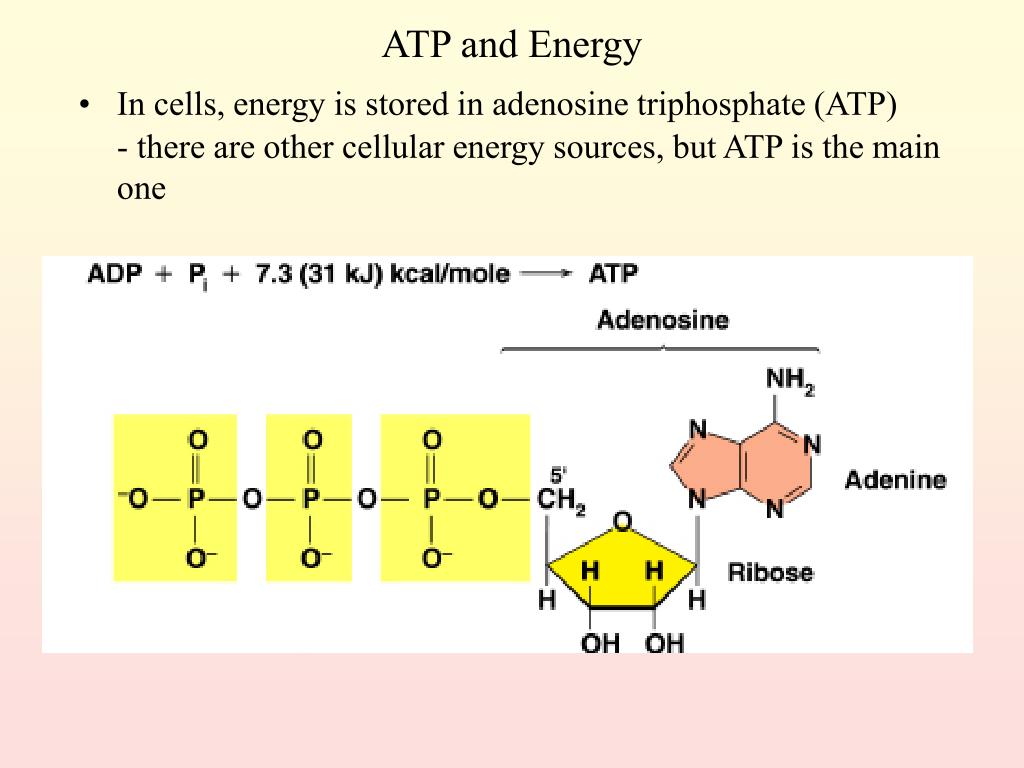
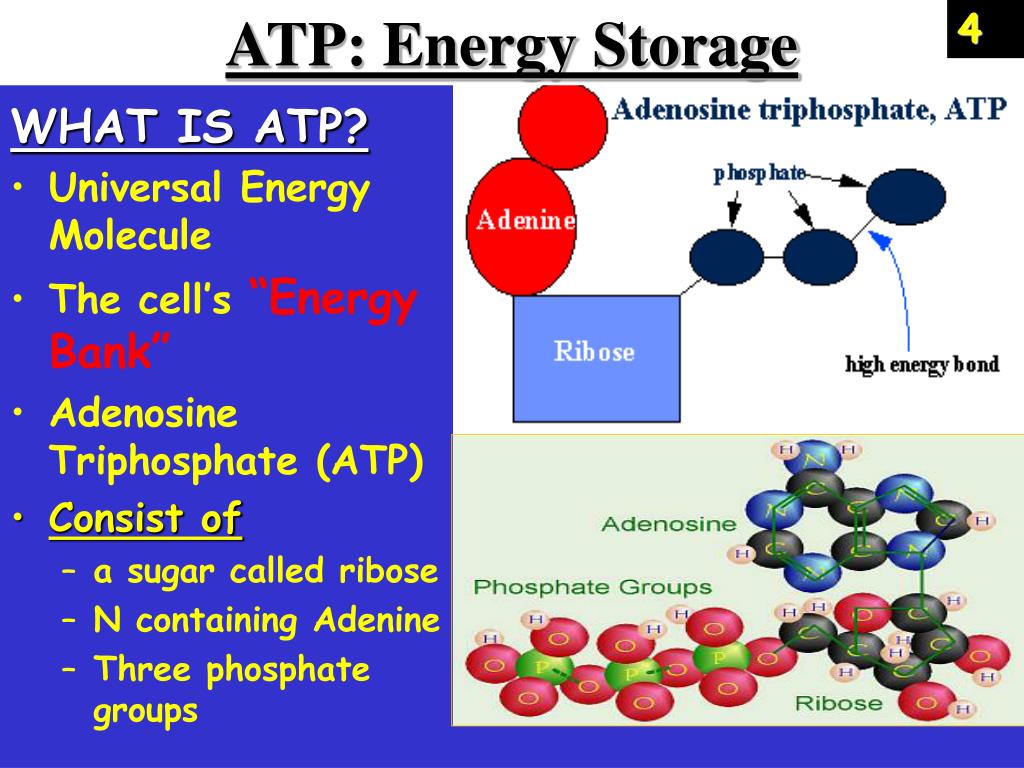
For years, the prevailing notion suggested that the high-energy phosphate bonds in ATP (adenosine triphosphate) held the key to cellular energy. However, recent research using advanced spectroscopic techniques and computational modeling has revealed a different story.
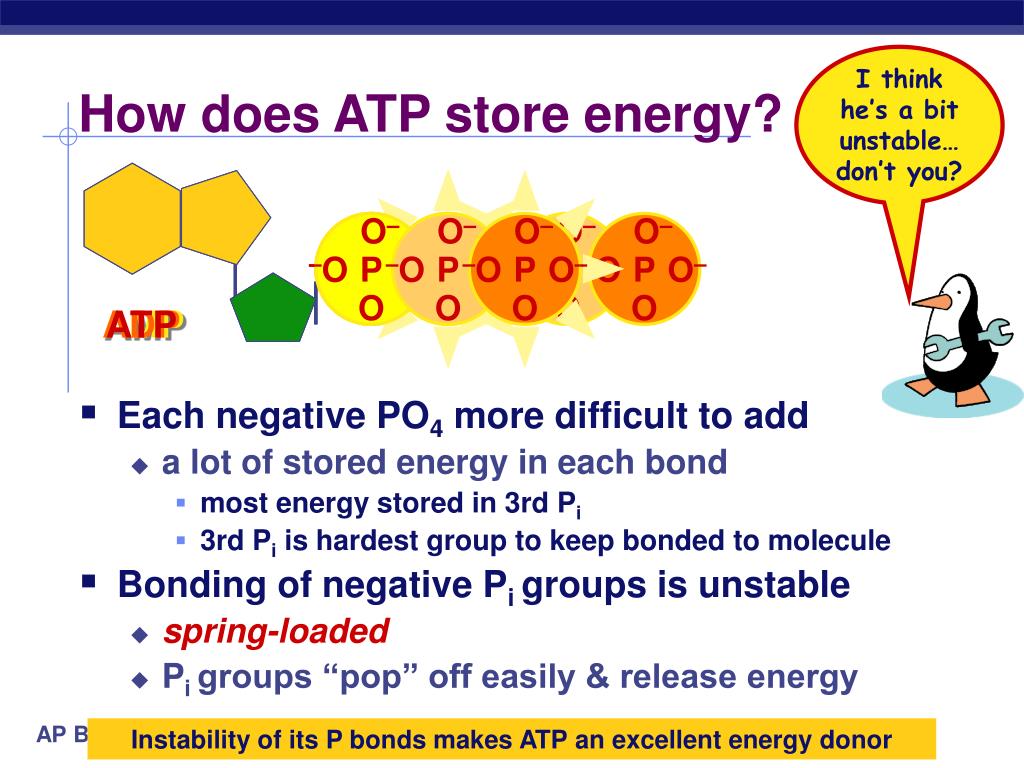
The true source of energy lies in the electrostatic repulsion between the negatively charged phosphate groups clustered together in the ATP molecule. These groups, due to their negative charges, naturally repel each other, creating a state of high potential energy.
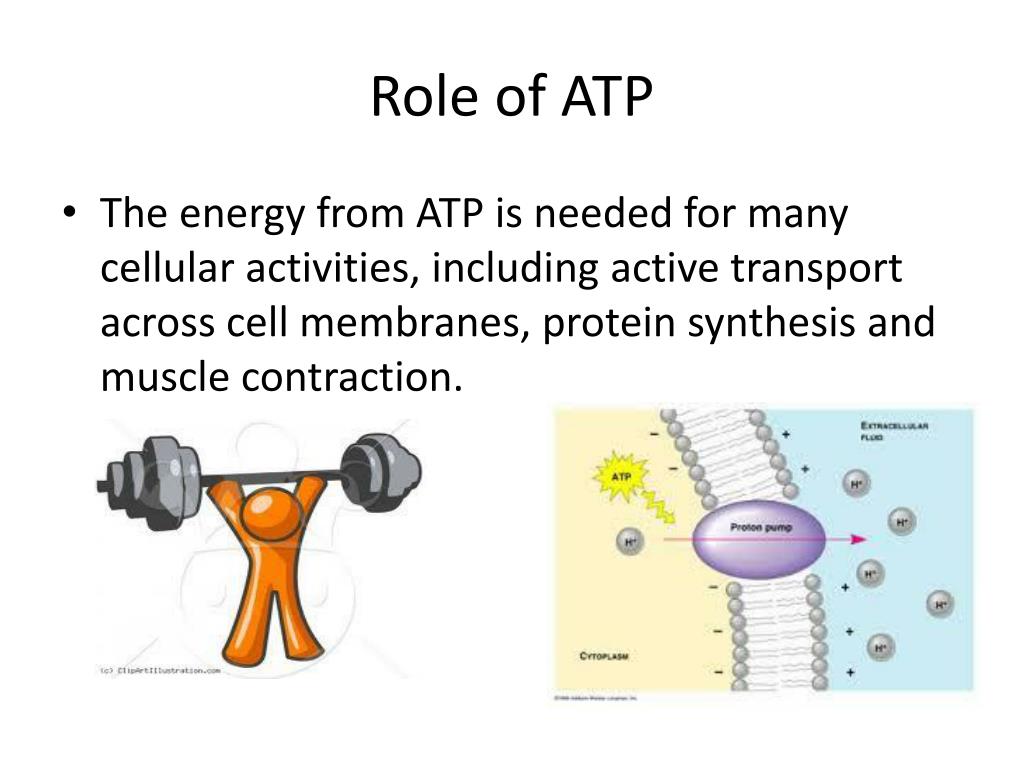
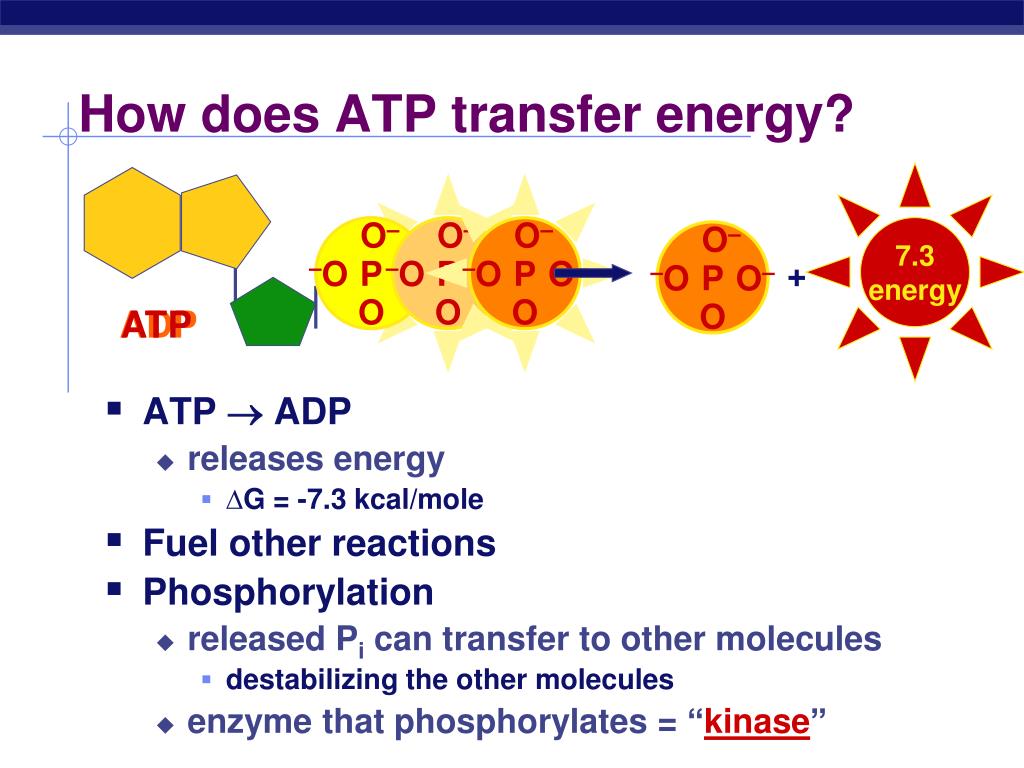
When one phosphate group is cleaved off (hydrolyzed), the repulsion is reduced, releasing energy that the cell can then use to perform work.
Where the Energy Resides: A Molecular Perspective
The energy isn't localized to a specific bond. It is distributed throughout the entire phosphate tail of the ATP molecule.
Hydrolysis of ATP reduces the charge density, allowing the remaining phosphate groups to relax into a lower energy state.
Think of it like a compressed spring: the energy is stored in the compression itself, not in any particular part of the spring's metal.
The "Who" Behind the Revelation
A collaborative team of researchers from several universities and research institutions spearheaded this work. Key figures include Dr. Emily Carter at Princeton University and Dr. Ken Dill at Stony Brook University, whose computational expertise was critical.
The research was also supported by grants from the National Institutes of Health (NIH) and the National Science Foundation (NSF), highlighting the importance of publicly funded scientific inquiry.
Their combined efforts brought together advanced spectroscopic techniques and cutting-edge computational modeling to unravel this biological mystery.
The "How" of the Experiment
Researchers employed sophisticated techniques such as X-ray absorption spectroscopy and molecular dynamics simulations. These allowed them to probe the electronic structure of ATP and track the movements of atoms and electrons during hydrolysis.
X-ray absorption spectroscopy provided detailed information about the energy levels of the phosphate groups. Molecular dynamics simulations enabled scientists to visualize the dynamic changes in the ATP molecule during the hydrolysis process.
By combining these methods, scientists were able to demonstrate conclusively that electrostatic repulsion, not bond energy, drives ATP's energy release.
Implications for the Future
This finding has profound implications for fields ranging from drug discovery to synthetic biology. Now that scientists understand the mechanism of energy release at a molecular level, they can design more effective drugs that target specific cellular processes.
For instance, understanding the charge distribution during ATP hydrolysis can guide the development of novel enzyme inhibitors.
Additionally, this knowledge can be used to create artificial systems that mimic the energy transfer processes found in living cells, potentially leading to new technologies in bioenergy and biomaterials.
Next Steps and Ongoing Developments
The research team is now focusing on exploring how this understanding can be applied to develop new cancer therapies. They are particularly interested in designing drugs that can disrupt the energy supply of cancer cells, thus preventing their uncontrolled growth.
Further studies are also underway to investigate the role of metal ions, such as magnesium, in modulating the electrostatic repulsion within ATP.
These ongoing investigations promise to further refine our understanding of ATP's energy dynamics and unlock new possibilities for manipulating cellular energy at the molecular level.