Which Change Of State Involves A Release Of Energy

The principles governing energy and matter are fundamental to understanding our world. Among these, the concept of phase transitions, or changes of state, plays a crucial role in various natural phenomena and technological applications. While some phase transitions require an input of energy, others release it, influencing everything from weather patterns to industrial processes.
This article delves into the specific change of state that involves a release of energy. Understanding the energy dynamics of phase transitions is essential for various fields, including climate science, materials engineering, and even culinary arts.
The Nut Graf: Exothermic Phase Transitions
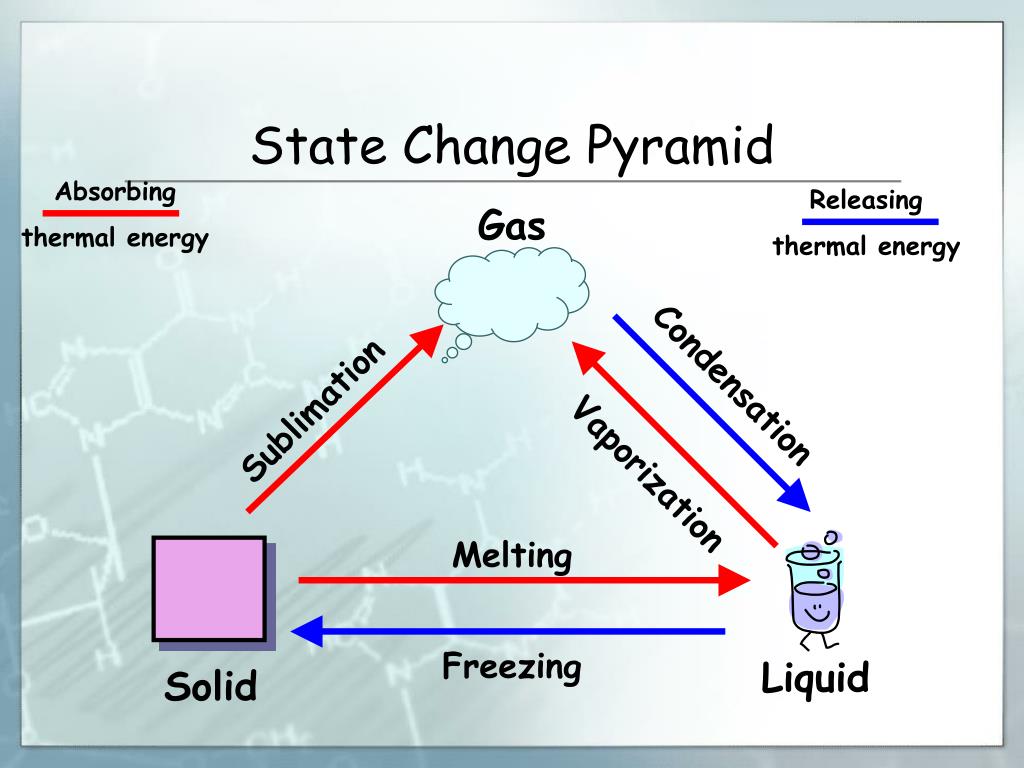
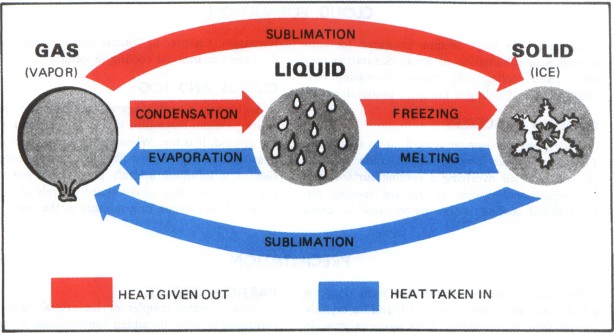
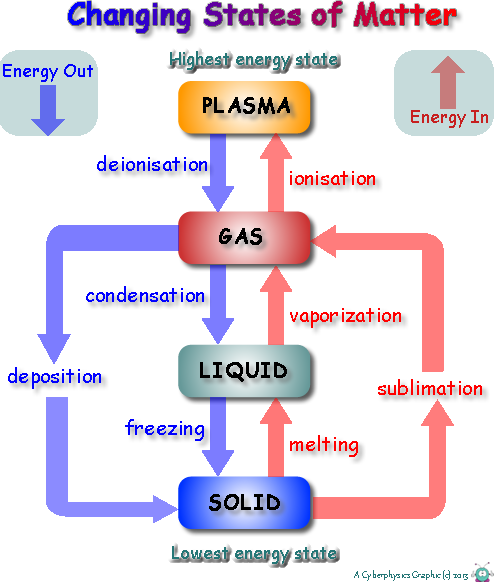
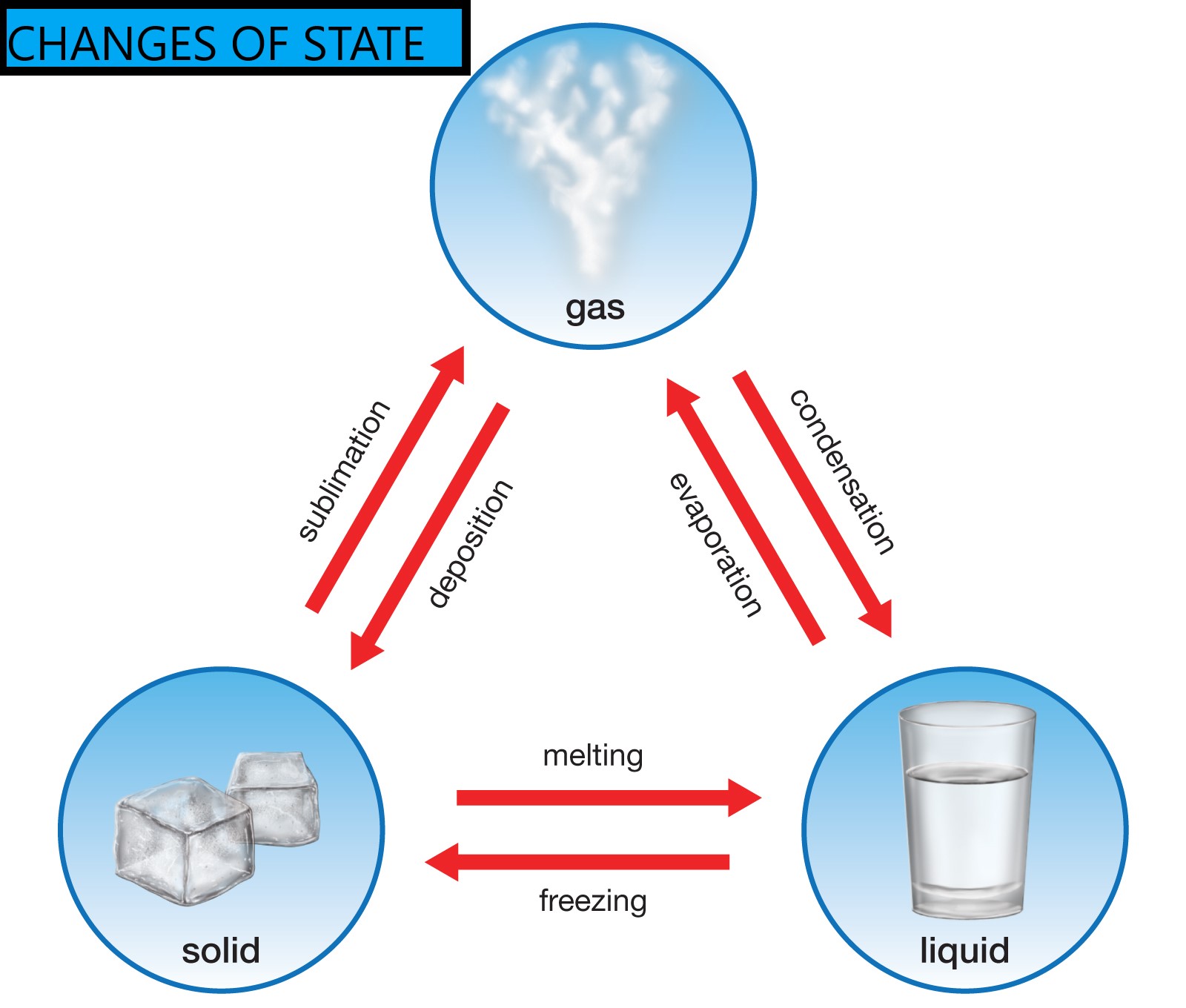
The change of state that releases energy is any transition from a higher energy state to a lower energy state. This includes condensation, freezing, and deposition. These processes are called exothermic phase transitions because they release energy into the surrounding environment, typically in the form of heat.
Condensation: Gas to Liquid
Condensation is the process by which a gas transforms into a liquid. Think of water vapor in the air turning into liquid water droplets on a cold surface. As gas molecules transition to a liquid state, they lose kinetic energy, which is released as heat.
This release of energy is why condensation feels warm to the touch under certain conditions. The energy that was previously used to keep the substance in its gaseous state is liberated as the molecules become more tightly packed in the liquid phase.
Freezing: Liquid to Solid
Freezing is the process by which a liquid transforms into a solid. A common example is water turning into ice. As a liquid transitions into a solid state, its molecules lose kinetic energy and become more ordered.
This organization and reduction in molecular motion release energy in the form of heat. Although the process appears cold to an observer, the water molecules themselves are releasing energy as they transition into the more stable, solid ice structure.
Deposition: Gas to Solid
Deposition is the phase transition in which a gas transforms directly into a solid, without passing through the liquid phase. A prime example is the formation of frost on a cold winter morning.
As gaseous water vapor directly transitions into solid ice crystals, a significant amount of energy is released. This energy release is the combined energy change of both condensation and freezing, making deposition a particularly exothermic process.
The Role of Intermolecular Forces
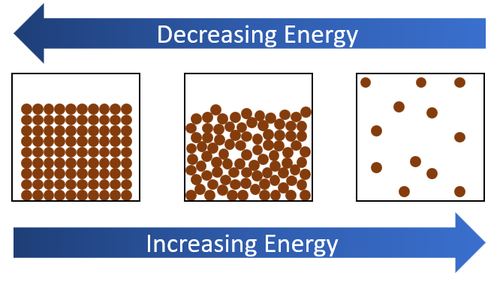
The release of energy during exothermic phase transitions is intimately linked to intermolecular forces. These forces are attractive forces that exist between molecules. In gases, these forces are relatively weak, allowing molecules to move freely.
However, in liquids and solids, these forces are stronger, holding the molecules closer together. As a substance transitions from a gas to a liquid or solid, the intermolecular forces become more dominant, leading to a release of energy as the molecules stabilize into a lower energy state.
Applications and Implications
Understanding exothermic phase transitions has practical applications across various fields. For instance, in meteorology, condensation plays a vital role in cloud formation and precipitation. The heat released during condensation can fuel the development of thunderstorms and hurricanes.
In industrial processes, exothermic phase transitions are utilized in refrigeration and air conditioning systems. The evaporation of a refrigerant absorbs heat, while its subsequent condensation releases heat, facilitating the transfer of thermal energy.
Counterpoint: Endothermic Phase Transitions
It's crucial to differentiate exothermic phase transitions from endothermic phase transitions. Endothermic processes, such as melting, boiling, and sublimation, require an input of energy to overcome intermolecular forces and transition to a higher energy state.
For example, melting ice requires energy to break the rigid structure of the ice crystal and allow the water molecules to move more freely as a liquid. This distinction highlights the duality of phase transitions and the intricate dance of energy gain and loss in the physical world.
The Broader Context: Thermodynamics
The study of phase transitions and their energy implications falls under the broader umbrella of thermodynamics. This branch of physics deals with the relationships between heat, work, and energy.
Understanding the thermodynamic principles governing phase transitions is essential for optimizing energy efficiency and developing sustainable technologies. From designing more efficient engines to creating advanced materials, thermodynamics provides a framework for harnessing the power of phase transitions.
Expert Opinion
According to Dr. Emily Carter, a professor of Chemical Engineering at Princeton University, "The understanding of exothermic phase transitions is crucial for designing energy-efficient systems. The ability to control and harness the energy released during these transitions is vital for various industrial applications, including power generation and chemical processing."
Dr. David Chandler, a renowned theoretical chemist at the University of California, Berkeley, emphasizes that "intermolecular forces are the key drivers behind these phase changes. The delicate balance between energy and entropy determines which phase is most stable under given conditions."
Future Directions
Research continues to explore the intricacies of phase transitions at the nanoscale and under extreme conditions. Scientists are investigating novel materials that exhibit unique phase transition behaviors, with potential applications in energy storage, sensing, and advanced electronics.
Furthermore, advancements in computational modeling are enabling researchers to predict and control phase transitions with greater precision. This could lead to the development of new technologies that leverage the energy released or absorbed during these transitions for a variety of applications.
Conclusion: Harnessing the Power of Phase Transitions
The change of state that releases energy—condensation, freezing, and deposition—plays a fundamental role in our world, influencing weather patterns, industrial processes, and even the formation of frost. By understanding the underlying principles of intermolecular forces and thermodynamics, scientists and engineers can harness the power of these transitions to create innovative technologies and address critical challenges in energy and sustainability.
As research continues to unravel the mysteries of phase transitions, we can expect even more exciting developments in the years to come, potentially transforming the way we use and manage energy.









.jpg)