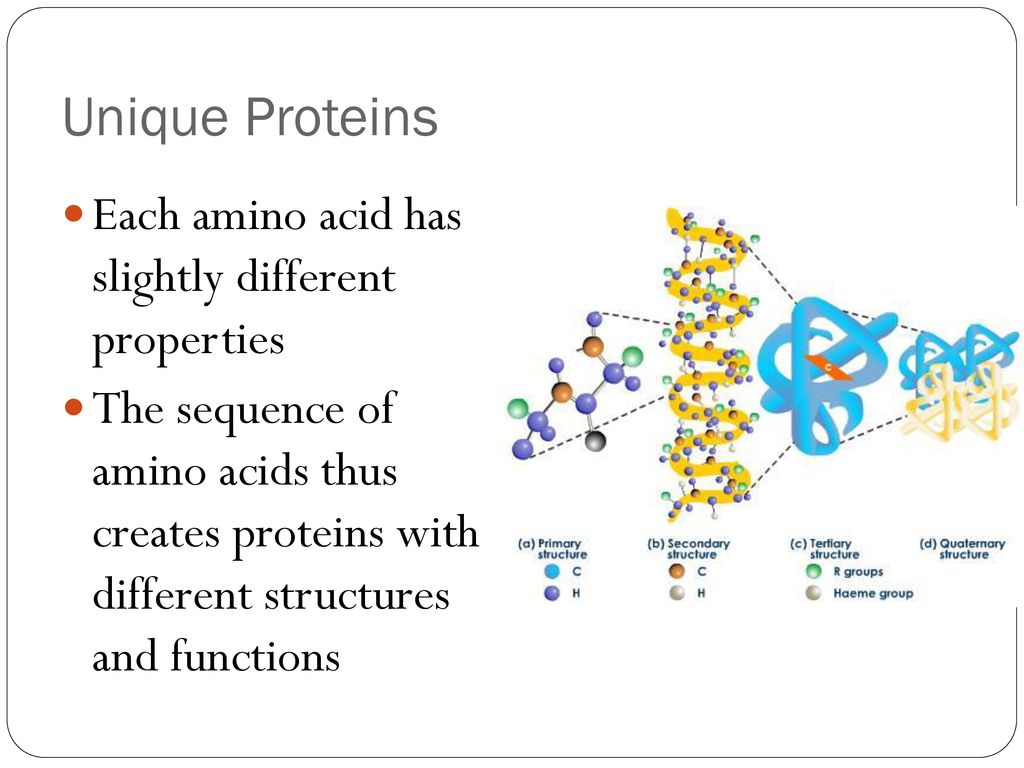
Which Component Makes Each Amino Acid Unique

The building blocks of life, amino acids, are fundamental to virtually every biological process. While sharing a common core structure, the subtle distinctions between them dictate the vast complexity and diversity of proteins. This seemingly simple difference – what makes each amino acid unique – holds the key to understanding everything from enzyme function to genetic code translation.
At the heart of this molecular individuality lies the side chain, also known as the R-group. This article delves into the crucial role of the side chain in defining the properties and functions of each of the 20 canonical amino acids, exploring how its structure, charge, and hydrophobicity contribute to the unique characteristics of proteins. Understanding this difference is paramount for advancements in fields ranging from drug discovery to materials science.
The Common Amino Acid Core
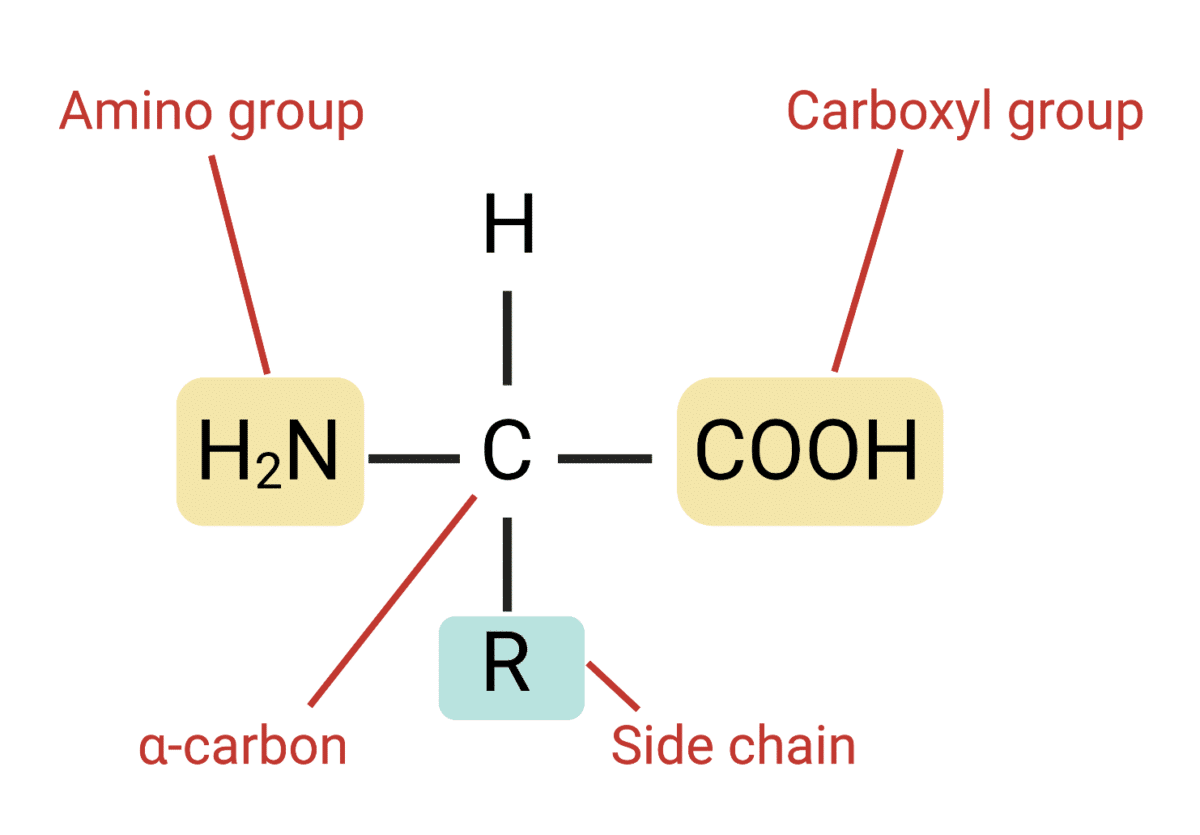
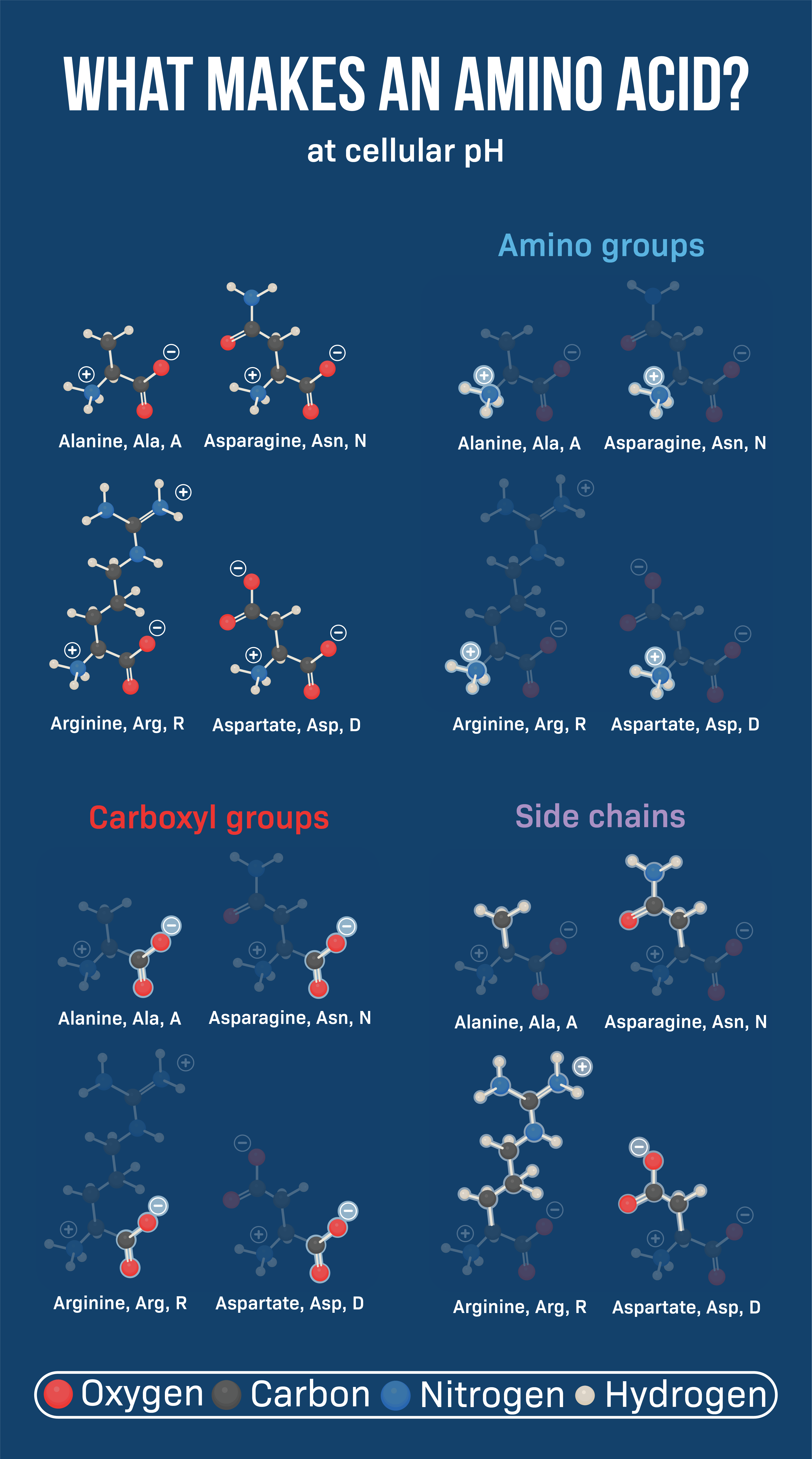
All amino acids share a central carbon atom, the alpha-carbon. Attached to this carbon are four groups: an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom (-H), and the side chain, or R-group. It's important to recognize that only the R-group is different.
The amino and carboxyl groups are responsible for the acid-base properties of amino acids. These groups enable the formation of peptide bonds, linking amino acids together to form polypeptide chains and ultimately, proteins.
The Defining Role of the R-Group
The R-group, projecting outward from the alpha-carbon, is the differentiating factor. This component dictates the amino acid's size, shape, reactivity, and interaction with other molecules.
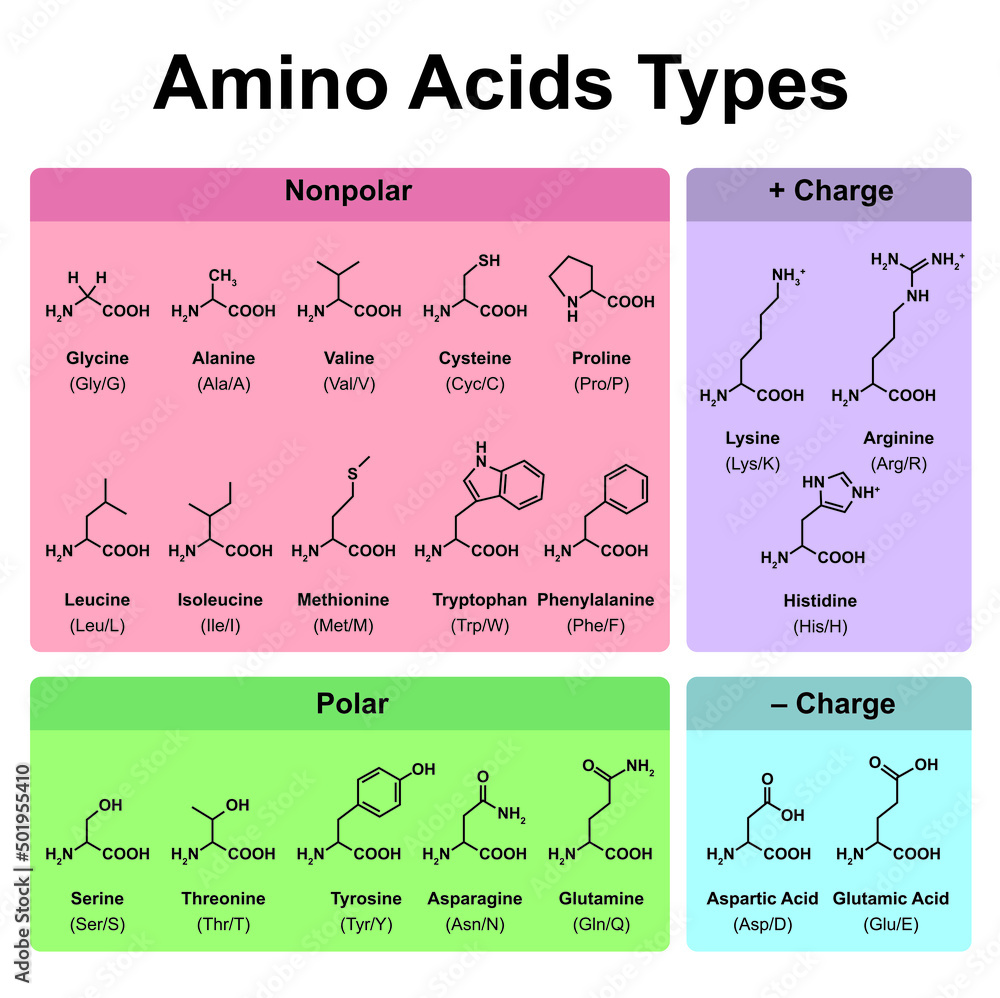
The 20 standard amino acids are categorized based on the properties of their R-groups. These properties include size, shape, charge, hydrophobicity (affinity for water), and hydrogen-bonding capacity.
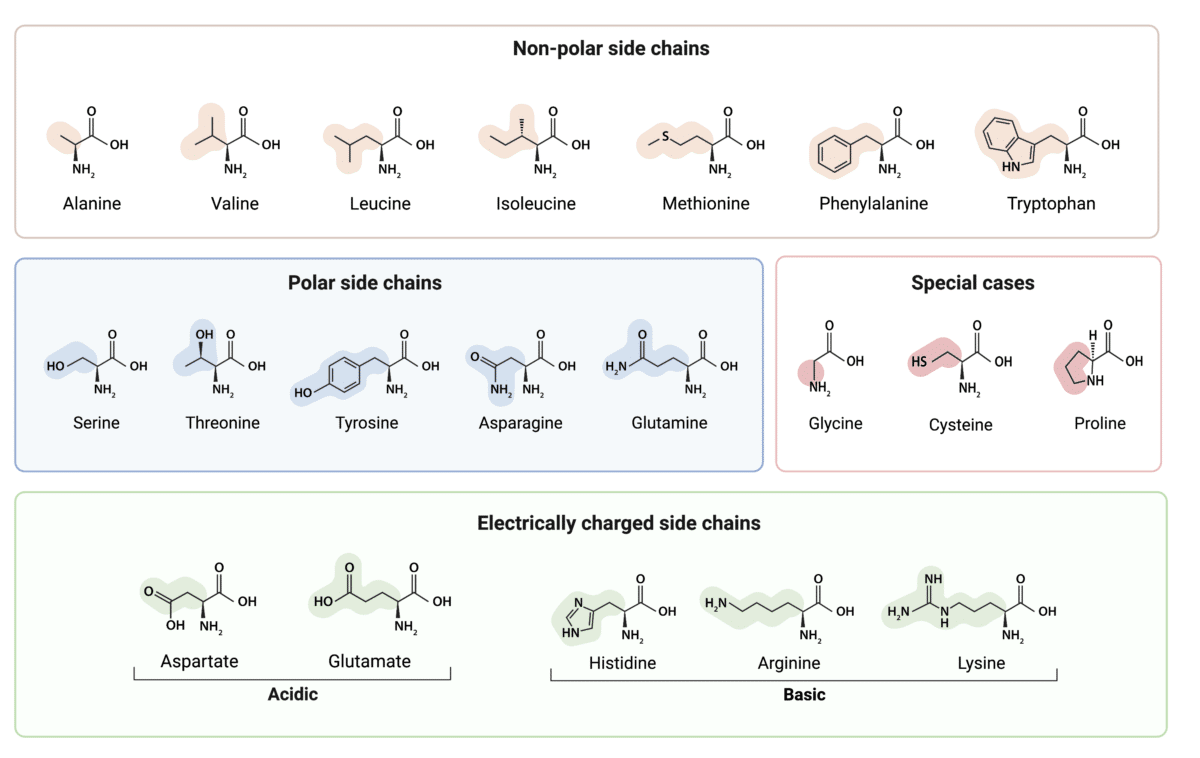
Categorization by Side Chain Properties
One of the primary classifications is based on the polarity of the R-group. Amino acids are broadly divided into nonpolar (hydrophobic), polar (hydrophilic), acidic (negatively charged), and basic (positively charged) categories. This categorization is critical because it influences how proteins fold and interact within cellular environments.
Nonpolar, Hydrophobic Amino Acids: These amino acids have side chains that are primarily composed of hydrocarbons. They tend to cluster together in the interior of proteins, away from the aqueous environment.
Examples include alanine, valine, leucine, isoleucine, phenylalanine, tryptophan, and methionine. Glycine, while technically having a single hydrogen atom as its side chain, is often grouped here due to its nonpolar nature.
Polar, Hydrophilic Amino Acids: These have side chains capable of forming hydrogen bonds with water. This increased solubility makes them more compatible with the aqueous environment of the cell.
Serine, threonine, cysteine, tyrosine, asparagine, and glutamine are examples. The presence of hydroxyl (-OH), thiol (-SH), or amide (-CONH2) groups enables them to form these hydrogen bonds.
Acidic (Negatively Charged) Amino Acids: These amino acids contain carboxyl groups in their side chains, giving them a negative charge at physiological pH. Aspartic acid (aspartate) and glutamic acid (glutamate) fall into this category.
The negative charge enables them to form ionic bonds with positively charged amino acids or other molecules.
Basic (Positively Charged) Amino Acids: These possess positively charged side chains at physiological pH. Lysine, arginine, and histidine are the three basic amino acids.
The positive charge allows them to interact with negatively charged molecules, DNA, or other proteins. Histidine's side chain can be protonated or deprotonated near physiological pH, making it crucial for many enzymatic reactions.
The Side Chain's Impact on Protein Structure and Function
The sequence of amino acids and the properties of their side chains dictate the three-dimensional structure of a protein. The hydrophobic effect, driven by the tendency of nonpolar side chains to avoid water, is a major driving force behind protein folding.
The interactions between polar and charged side chains further refine the protein's shape. Hydrogen bonds, ionic bonds, and van der Waals forces play vital roles in stabilizing the structure.
The specific arrangement of amino acid side chains in the active site of an enzyme is critical for its catalytic activity. These side chains participate directly in substrate binding and chemical transformations.
Even subtle changes in the amino acid sequence, such as a single amino acid substitution, can dramatically alter protein structure and function. This can lead to a variety of diseases or altered physiological responses.
Beyond the Canonical 20
While the 20 canonical amino acids are the primary building blocks of proteins, some amino acids are modified after the protein is synthesized. These post-translational modifications further expand the diversity of protein function. Examples include phosphorylation, glycosylation, and hydroxylation.
Some organisms also incorporate non-canonical amino acids into their proteins during translation. Selenocysteine and pyrrolysine, for instance, are genetically encoded in certain organisms and incorporated using specialized tRNA molecules.
Future Directions
Research continues to explore the diverse roles of amino acid side chains in protein structure, function, and interactions. Scientists are utilizing computational methods to predict protein structure and design novel proteins with specific properties.
Understanding the subtle effects of amino acid substitutions is also critical for developing personalized medicine approaches. Identifying genetic variants that alter protein function can lead to tailored treatments for individual patients. Further, the field of synthetic biology is leveraging this knowledge to engineer novel proteins and enzymes with enhanced or entirely new capabilities.
The study of amino acid side chains remains a cornerstone of modern biology. By unraveling the intricate connections between side chain properties and protein function, we are unlocking new avenues for understanding life's complexity and developing innovative solutions to global challenges.





+Alpha+carbon+(C).jpg)