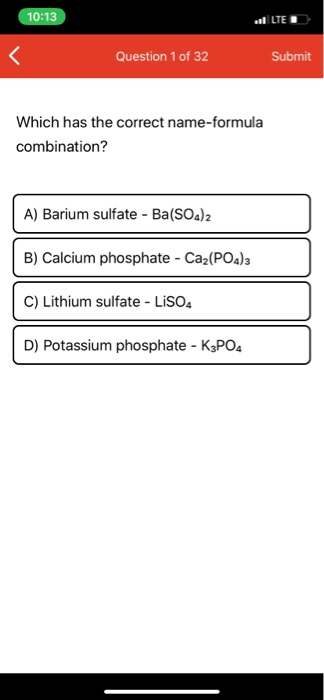
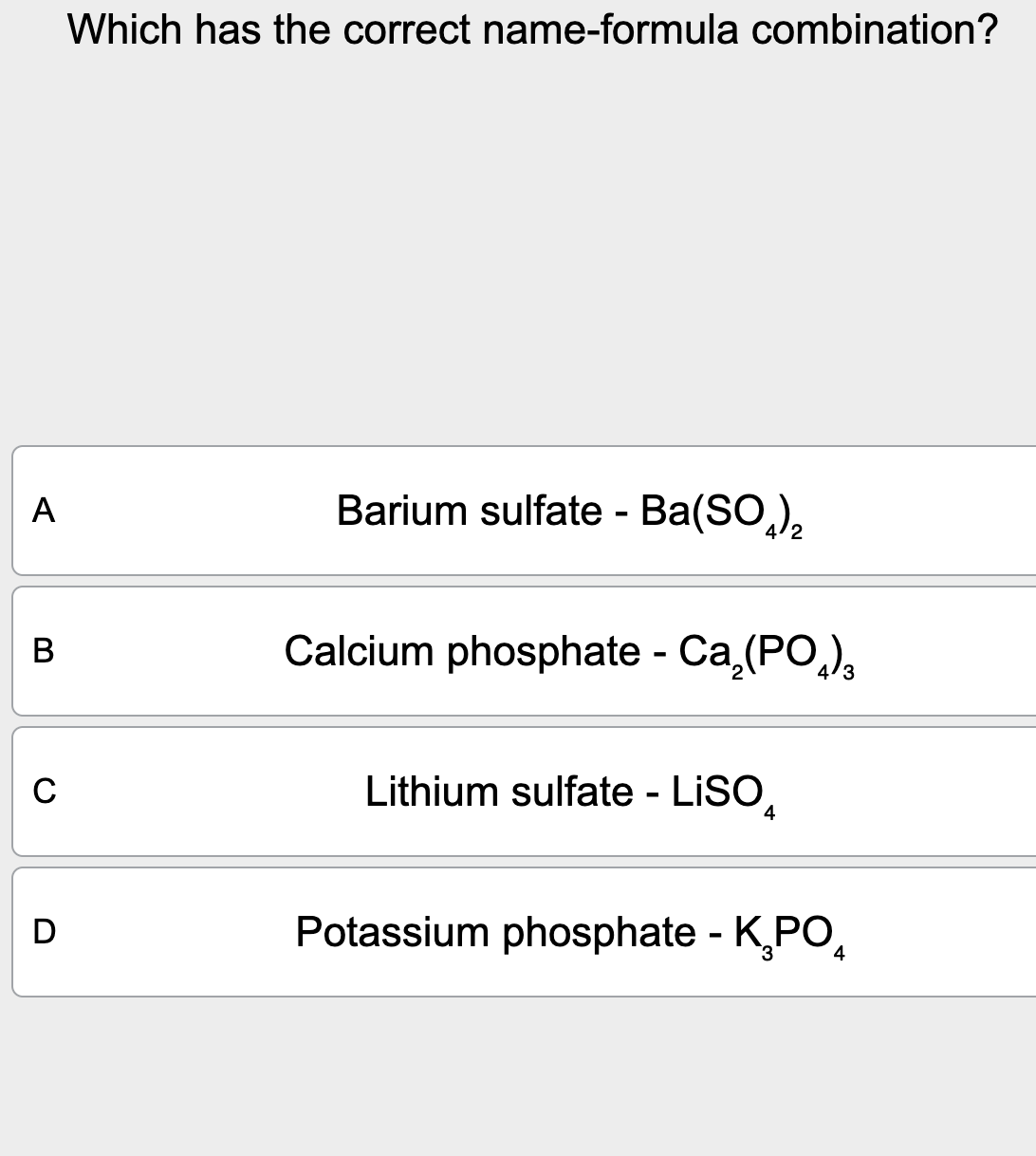
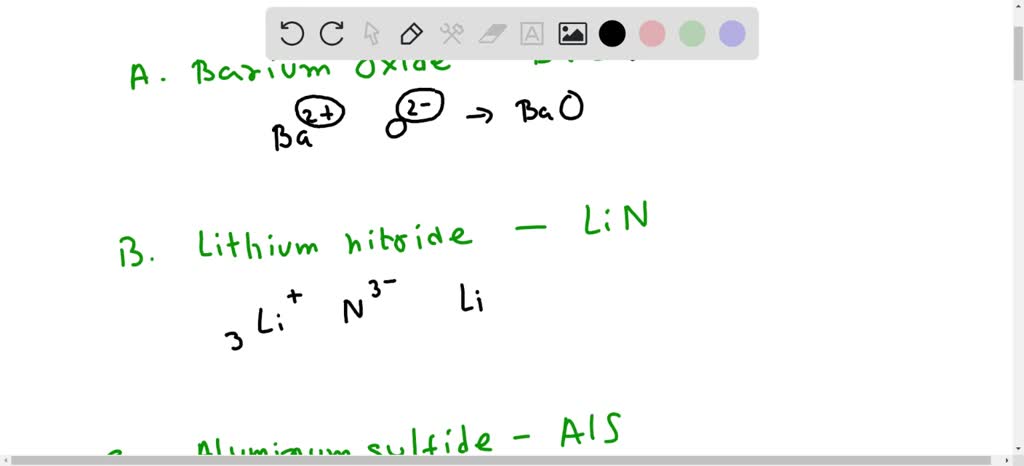
Which Has The Correct Name-formula Combination

Imagine a bustling chemistry classroom, filled with the aroma of beakers and the excited chatter of students. Sunlight streams through the windows, illuminating the periodic table hanging proudly on the wall. In the center of it all, a friendly debate rages on: Is it sodium chloride (NaCl) or chlorine sodium (ClNa)? This is not just about memorization; it's about understanding the fundamental principles that govern how elements interact and form the world around us.
This article dives into the core principles behind chemical nomenclature and formula writing. We'll explore why specific names and formulas are assigned to compounds, ensuring clarity and consistency in the scientific community. Understanding these rules is critical not only for chemistry students but also for anyone interested in the language of science and the composition of the materials they encounter daily.
The Language of Chemistry: Nomenclature and Formulas
At its heart, chemistry is a language, and like any language, it has its own set of rules and grammar. Chemical nomenclature is the system of naming chemical compounds, while chemical formulas represent the types and numbers of atoms present in a molecule or compound. These are not arbitrary choices; they are governed by established conventions.
The International Union of Pure and Applied Chemistry (IUPAC) is the globally recognized authority on chemical nomenclature. IUPAC develops and maintains standards for naming chemical compounds to ensure that scientists worldwide can communicate clearly and unambiguously. This standardization is vital for research, education, and industrial applications.
Why Order Matters
In writing chemical formulas, the order of elements is not random. For ionic compounds, the cation (positively charged ion) is always written first, followed by the anion (negatively charged ion). This convention stems from the historical understanding of how these compounds are formed and their electrical properties.
For example, consider sodium chloride (NaCl), commonly known as table salt. Sodium (Na) forms a cation (Na+), while chlorine (Cl) forms an anion (Cl-). Therefore, the correct formula is NaCl, reflecting the ionic nature and charge balance of the compound.
Writing it as ClNa would be incorrect because it implies that chlorine is the positively charged ion and sodium is the negatively charged ion, which is not the case. This highlights the importance of following established conventions to accurately represent the chemical composition.
Naming Conventions: A Closer Look
The naming of chemical compounds follows a similar set of rules. For ionic compounds, the name of the cation remains unchanged, while the name of the anion is modified to end in "-ide." For instance, in sodium chloride, "sodium" remains as is, and "chlorine" becomes "chloride."
In covalent compounds (compounds formed by sharing electrons), prefixes are used to indicate the number of atoms of each element present. For example, carbon dioxide (CO2) indicates one carbon atom and two oxygen atoms. These prefixes, such as "di-", "tri-", and "tetra-", provide crucial information about the molecular structure.
These naming conventions are essential for accurately identifying and differentiating between various chemical compounds. Without a standardized system, confusion and misinterpretation would be rampant in scientific communication.
Exceptions and Nuances
While the basic rules are straightforward, there are exceptions and nuances to chemical nomenclature. Transition metals, for example, can form multiple ions with different charges. In these cases, Roman numerals are used in the name to indicate the charge of the metal ion. For instance, iron(II) chloride (FeCl2) contains iron with a +2 charge, while iron(III) chloride (FeCl3) contains iron with a +3 charge.
Polyatomic ions, which are ions composed of multiple atoms, also have specific names that must be memorized. Examples include sulfate (SO42-), nitrate (NO3-), and ammonium (NH4+). Knowing these polyatomic ions is crucial for naming and writing formulas for many common compounds.
Organic compounds, which contain carbon, have a complex but systematic nomenclature system based on the structure of the carbon backbone and the functional groups attached to it. The IUPAC nomenclature for organic compounds is extensive and requires a deeper understanding of organic chemistry principles.
Why It Matters: The Significance of Correct Nomenclature
Accurate chemical nomenclature and formula writing are not merely academic exercises; they are essential for safety, research, and industrial applications. Incorrectly naming or writing a formula could lead to confusion and potentially dangerous situations.
In the pharmaceutical industry, for example, the correct identification of chemical compounds is paramount. Administering the wrong medication due to misidentification could have severe consequences. Similarly, in chemical manufacturing, using the wrong chemical in a process could lead to explosions or the production of unwanted and potentially harmful byproducts.
The scientific literature relies heavily on accurate and consistent nomenclature. Researchers must be able to clearly and unambiguously describe the compounds they are working with to ensure that their findings can be replicated and validated by others. Accurate nomenclature also facilitates the indexing and retrieval of scientific information, making it easier for scientists to access relevant research.
"The importance of a clear and unambiguous chemical nomenclature cannot be overstated. It is the foundation upon which all chemical communication rests." - Linus Pauling, Nobel Laureate in Chemistry
Real-World Examples
Consider the case of water (H2O). The formula clearly indicates that each molecule of water contains two hydrogen atoms and one oxygen atom. Writing it as OH2 would be incorrect, even though the same elements are present. The correct formula reflects the molecular structure and properties of water.
Another example is sodium hydroxide (NaOH), a strong base commonly used in cleaning products. The formula indicates that it contains sodium (Na), oxygen (O), and hydrogen (H). If it were incorrectly written as NaHO, it would still contain the same elements but would imply a different molecular arrangement and potentially different chemical properties.
These examples illustrate that the order and arrangement of elements in a chemical formula are not arbitrary; they reflect the underlying chemical structure and properties of the compound.
Conclusion: A Universal Language
Understanding chemical nomenclature and formula writing is like learning a universal language that connects scientists and researchers around the world. It enables clear and unambiguous communication, facilitating collaboration and innovation.
While the rules may seem daunting at first, they are designed to provide a systematic and logical framework for describing the chemical world. By mastering these principles, we gain a deeper appreciation for the intricate beauty and complexity of chemistry.
So, the next time you see a chemical formula or name, remember that it is more than just a collection of symbols or words. It is a carefully constructed representation of the fundamental building blocks of our universe, a testament to the power of human curiosity and the quest to understand the world around us. Whether it is sodium chloride or water, the correct name-formula combination is a key to unlocking a deeper understanding of the world.