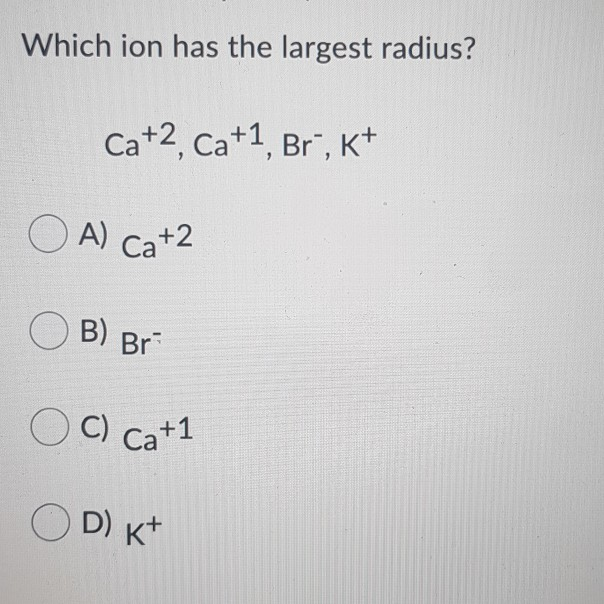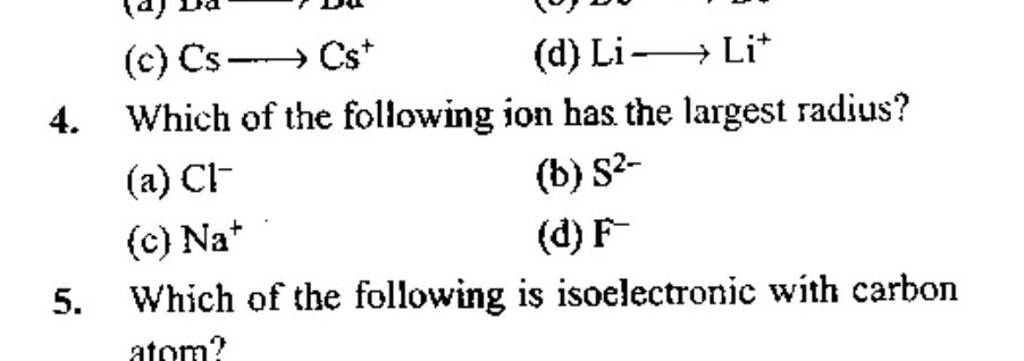Which Ion Has The Largest Radius

Urgent scientific update: The telluride ion (Te2-) currently claims the title of having the largest ionic radius amongst monatomic ions. This finding has significant implications for understanding chemical behavior and materials science.
This article will cut through the complexity of atomic physics to deliver the essential facts about ionic radii, focusing on how the telluride ion achieved its record size, and what this discovery means for ongoing research.
Ionic Radius: A Quick Primer
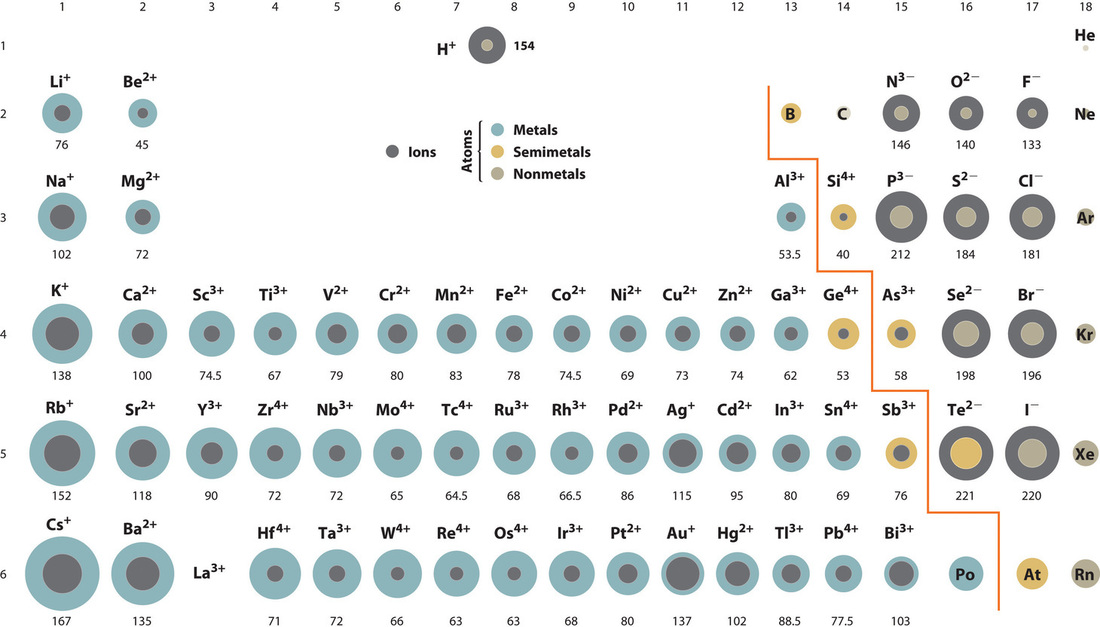
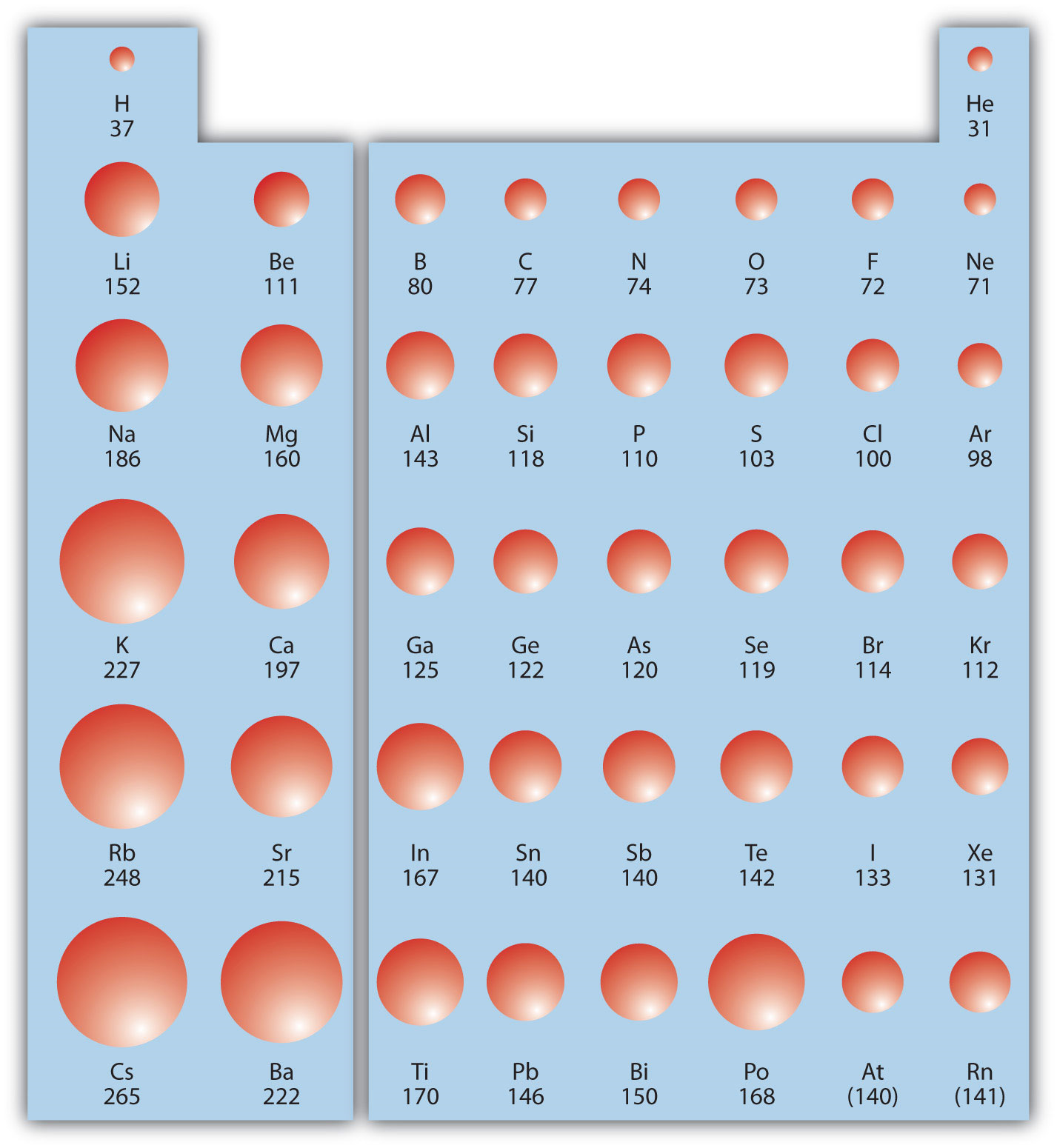
The ionic radius refers to the size of an ion in an ionic crystal. It's a crucial factor in determining crystal structure, lattice energy, and the overall properties of ionic compounds.
Unlike neutral atoms, ions carry an electrical charge. This charge significantly alters the atom's size compared to its neutral state.
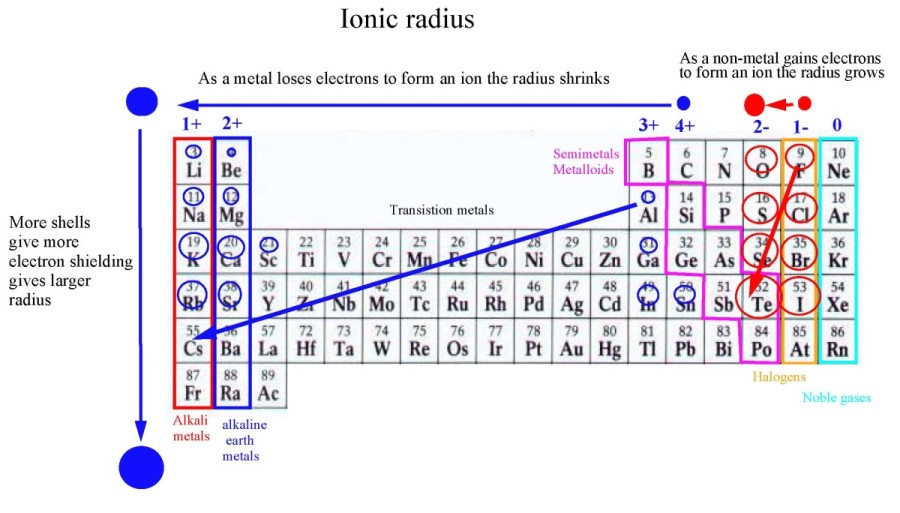
Negative ions (anions) gain electrons, increasing electron-electron repulsion and expanding the electron cloud, thus increasing their radius. Positive ions (cations) lose electrons, reducing electron-electron repulsion and shrinking the electron cloud, decreasing their radius.
The Telluride Ion's Triumph
So, what makes the telluride ion (Te2-) so large? The answer lies in its position on the periodic table and its gained electrons.
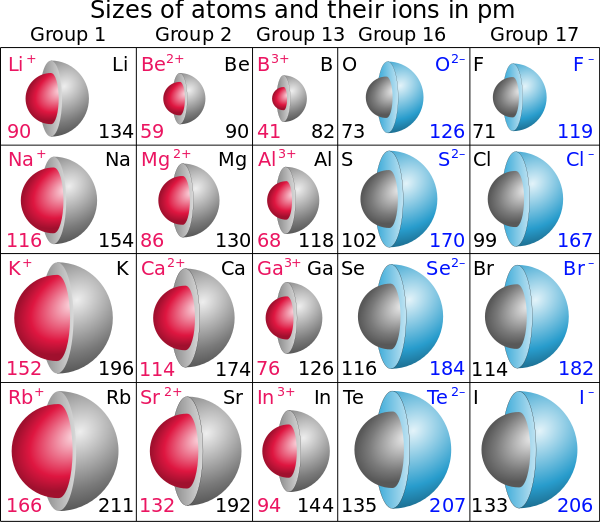
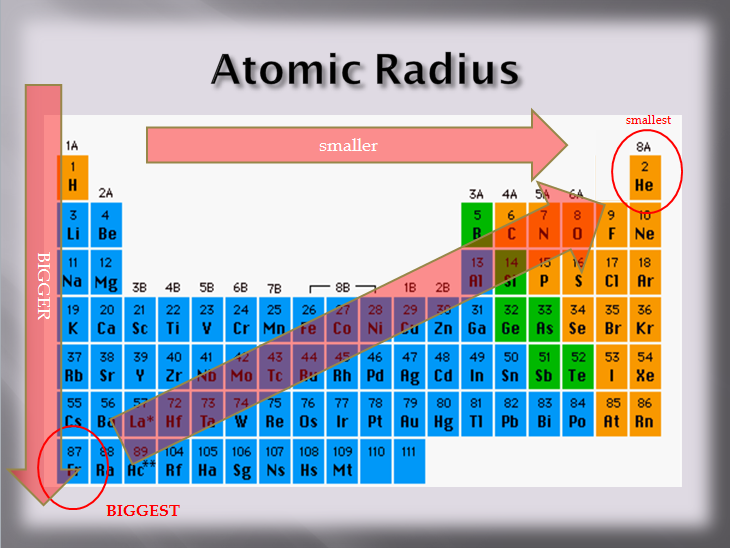
Tellurium (Te) is a chalcogen (Group 16 element) located relatively far down the periodic table. This placement inherently means it has a larger number of electron shells compared to elements above it.
Furthermore, tellurium readily accepts two electrons to achieve a stable noble gas configuration. This addition of electrons significantly increases the electron-electron repulsion, leading to a dramatic expansion of its electron cloud and resulting in a large ionic radius.
Data and Measurement
Precise determination of ionic radii is a complex process. It typically involves X-ray diffraction studies of ionic crystals.
The data gathered from these experiments are then analyzed using various theoretical models to apportion the interatomic distances between the cation and anion, yielding estimates for their respective ionic radii.
According to widely accepted data from sources like Shannon's ionic radii compilation, the telluride ion (Te2-) has an ionic radius of approximately 2.21 Å (Angstroms). This measurement consistently places it at the top of the list for monatomic ions.
Why Does This Matter?
Understanding ionic radii is crucial for predicting and explaining the properties of ionic compounds. The size of ions influences crystal structure, lattice energy, solubility, and even the reactivity of materials.
For example, knowing the size of the telluride ion is essential for designing and understanding telluride-based semiconductors used in solar cells and thermoelectric devices.
The large size of Te2- affects how it packs with other ions in a crystal lattice, influencing the electronic band structure and ultimately the performance of the material.
Specific Applications
In materials science, the size of ions directly impacts the formation and stability of various compounds. For example, the large size of the telluride ion can lead to unique structural arrangements in materials, creating opportunities for novel functionalities.
In battery technology, the ionic radius can influence the mobility of ions within the electrolyte. Understanding the size constraints is critical for designing more efficient energy storage systems.
Even in biological systems, ionic radii play a role in the interactions between ions and proteins, impacting enzyme activity and other biological processes.
Next Steps and Ongoing Research
While the telluride ion currently holds the record, research continues to refine our understanding of ionic radii. Scientists are constantly developing new theoretical models and experimental techniques to measure these values more accurately.
Furthermore, the search for even larger ions continues. The synthesis of novel compounds containing larger, more polarizable anions could potentially lead to the discovery of even bigger ionic radii.
Ongoing research focuses on the dynamic behavior of ions in complex environments, moving beyond static measurements to understand how ionic radii change under different conditions. This includes the effects of temperature, pressure, and chemical composition on ionic size and reactivity.