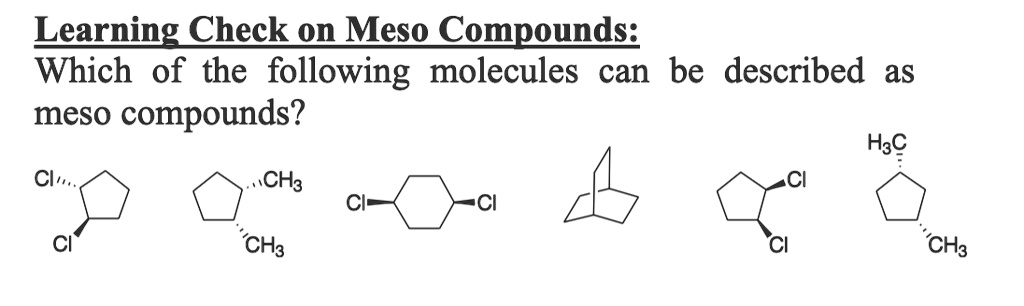
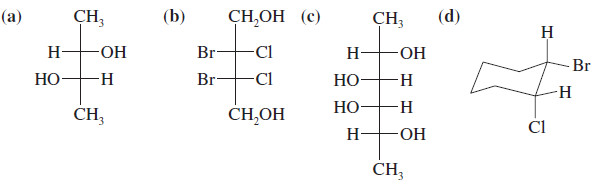
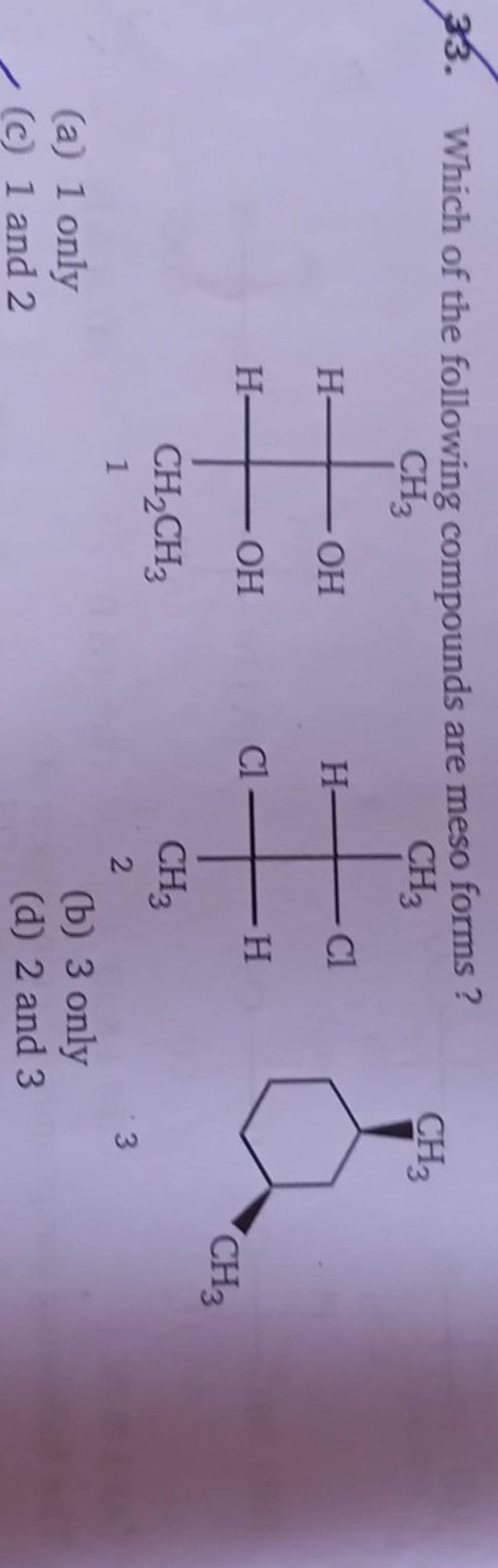
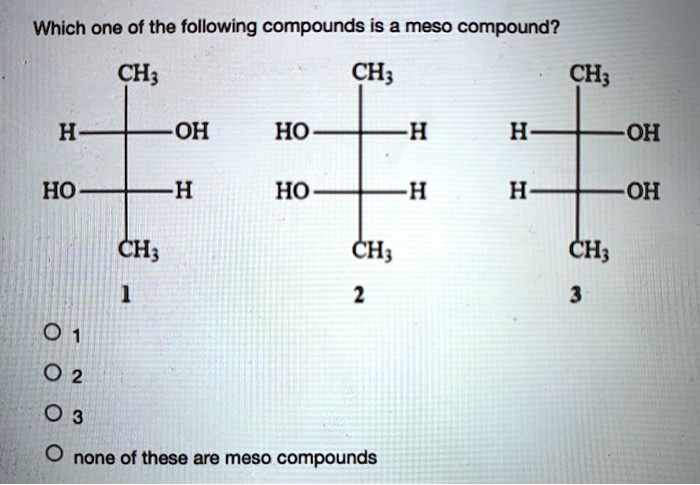
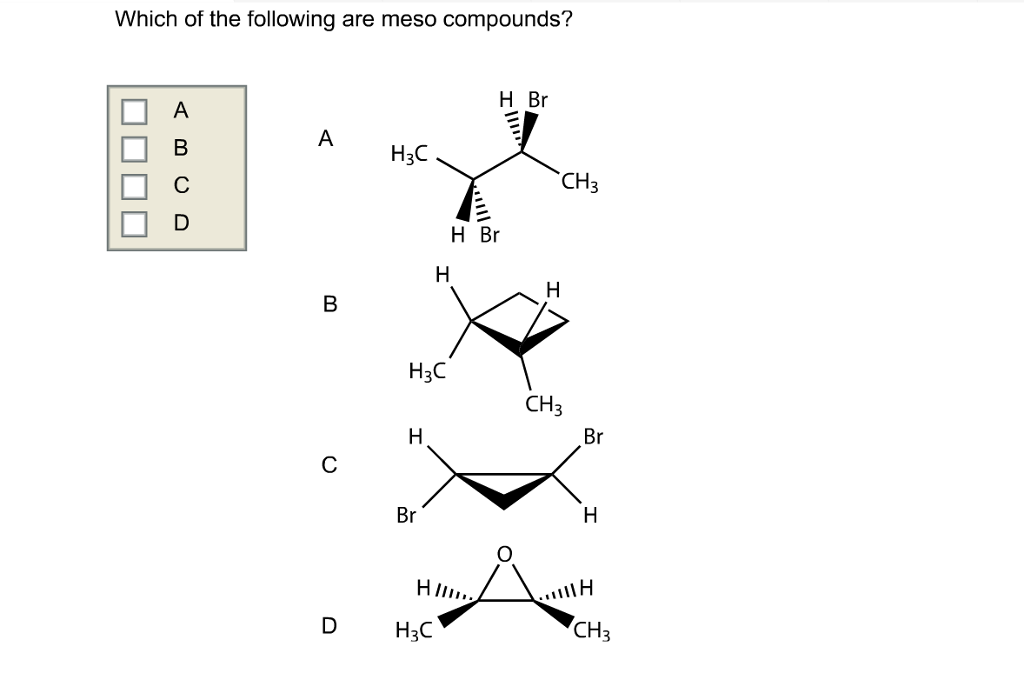
Which Of The Following Are Meso Compounds
The seemingly simple question, "Which of the following are meso compounds?" has become a surprisingly contentious battleground in undergraduate organic chemistry education. Students across the globe grapple with the concept, leading to widespread confusion and impacting their understanding of stereochemistry, a fundamental building block for advanced chemistry topics.
This persistent difficulty highlights deeper issues within chemical education: the complexities of three-dimensional thinking, the limitations of two-dimensional representations, and the varying pedagogical approaches employed by instructors. Understanding meso compounds requires a solid grasp of chirality, symmetry, and stereoisomers, concepts that many find abstract and challenging.
Defining the Meso Compound
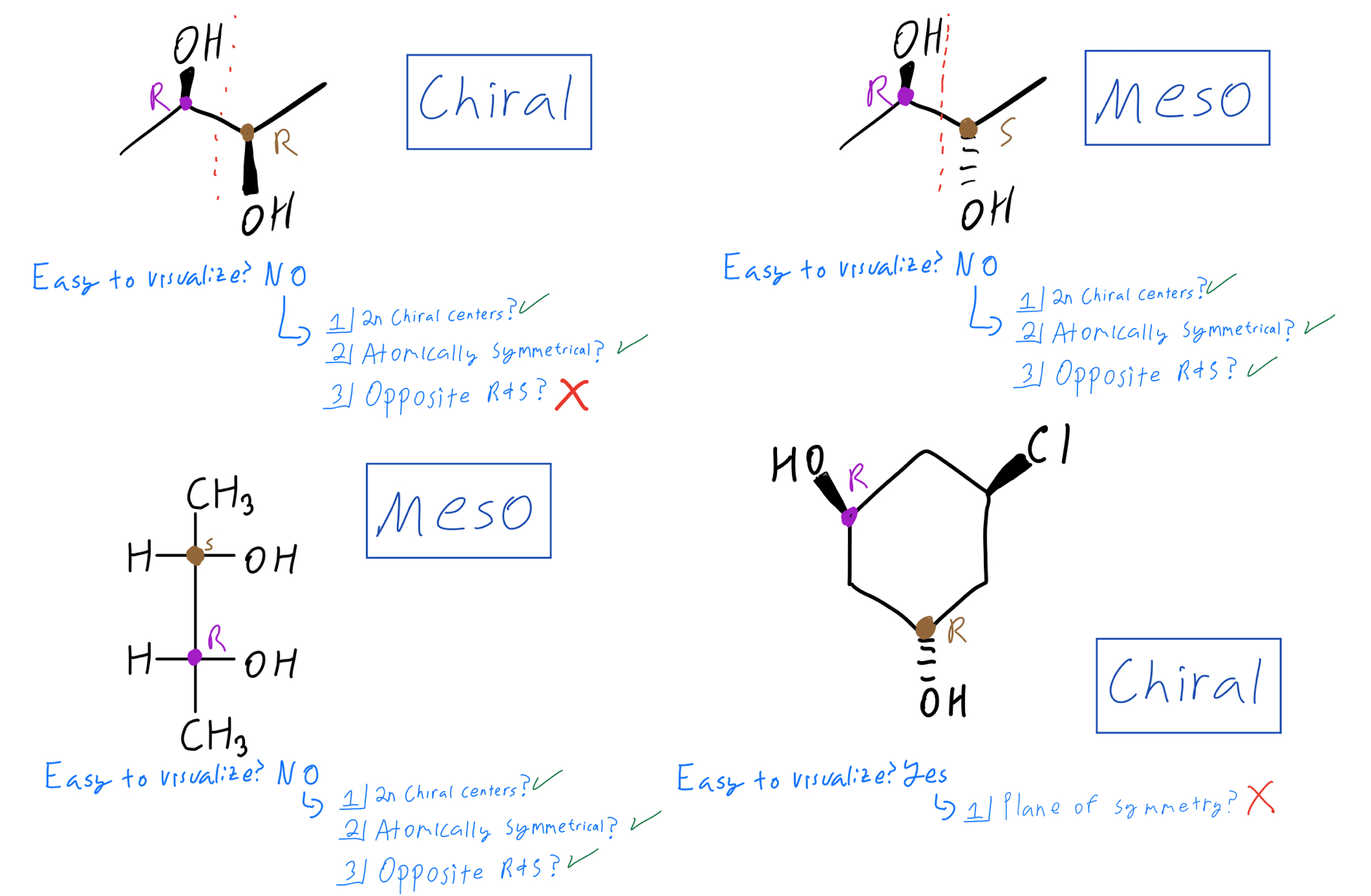
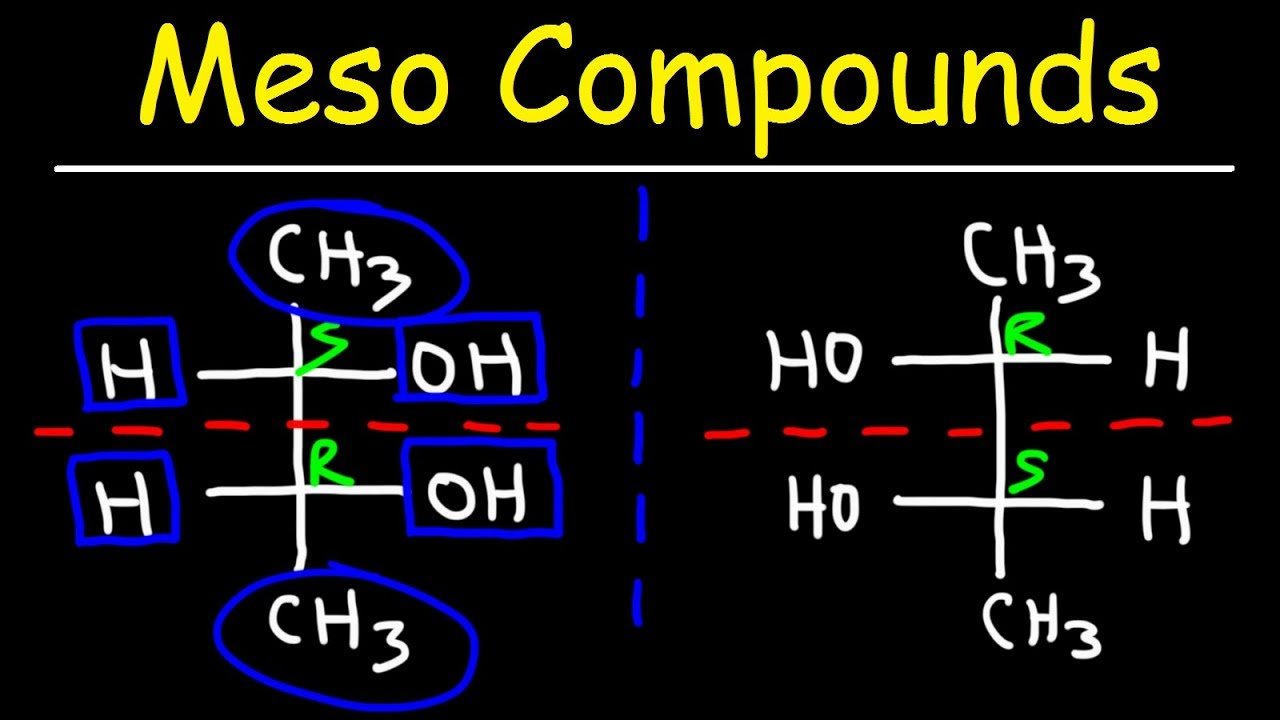
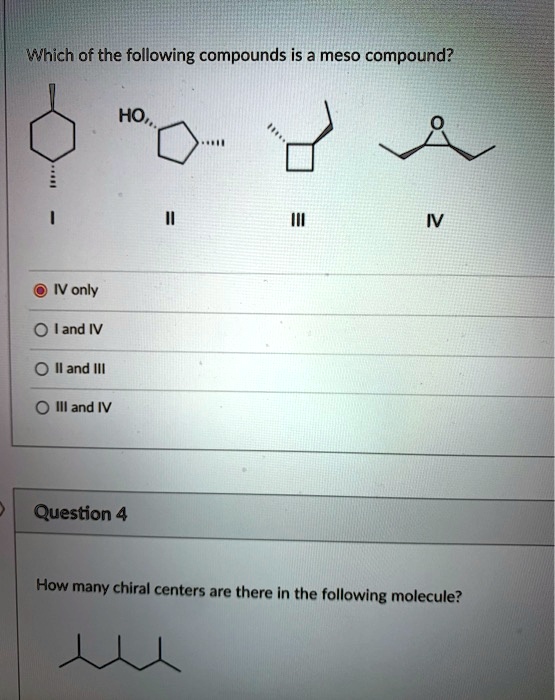
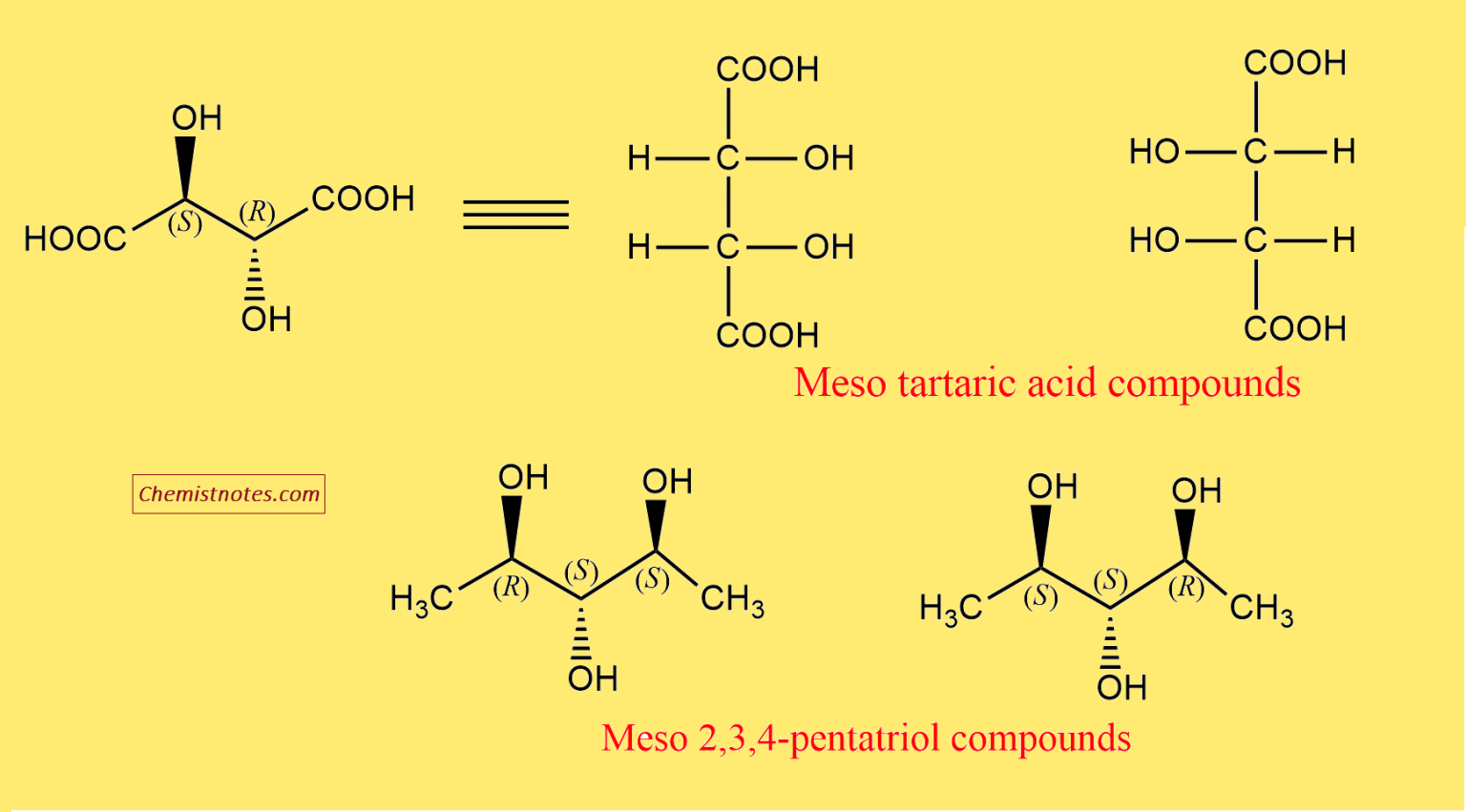
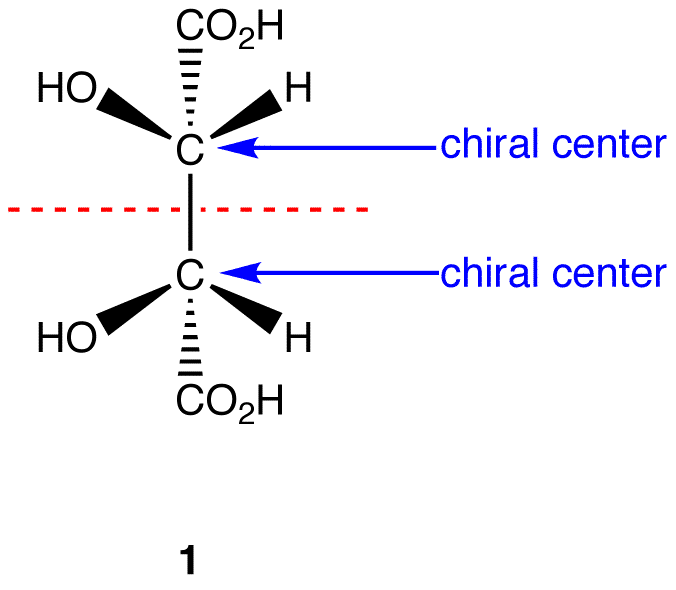
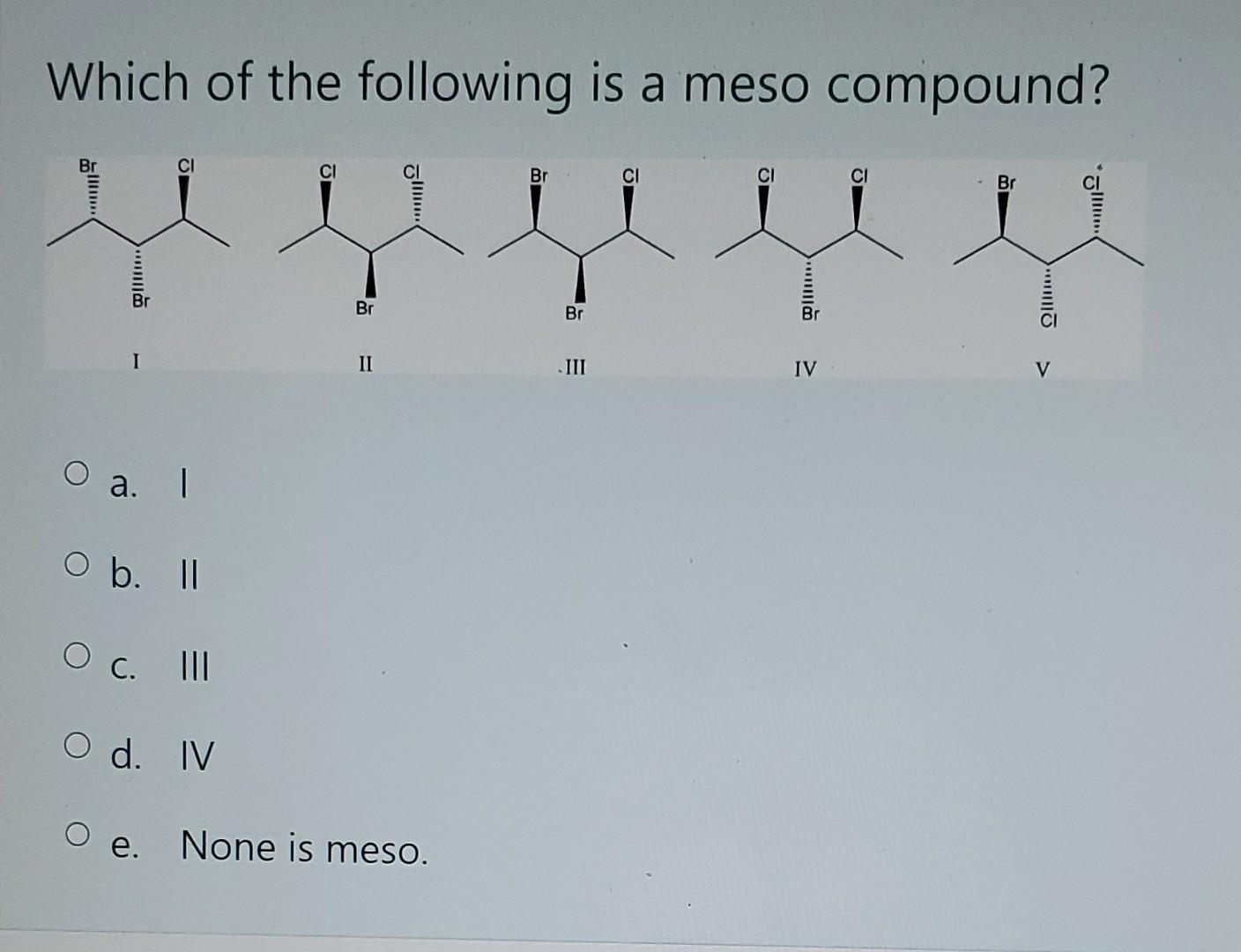
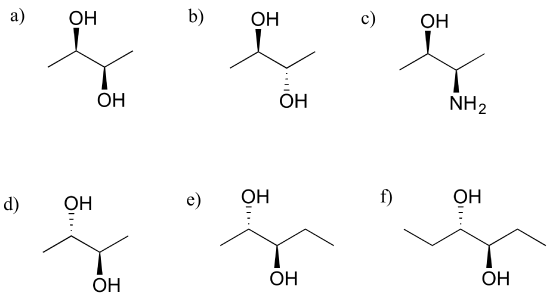
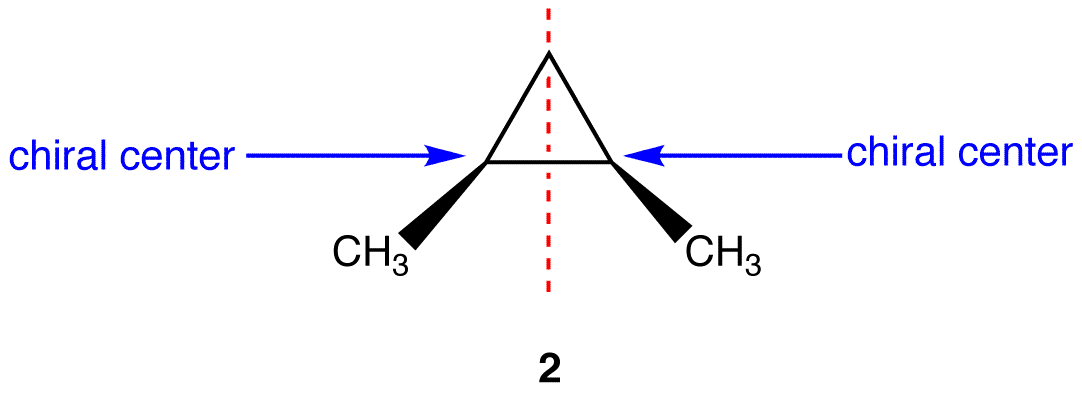
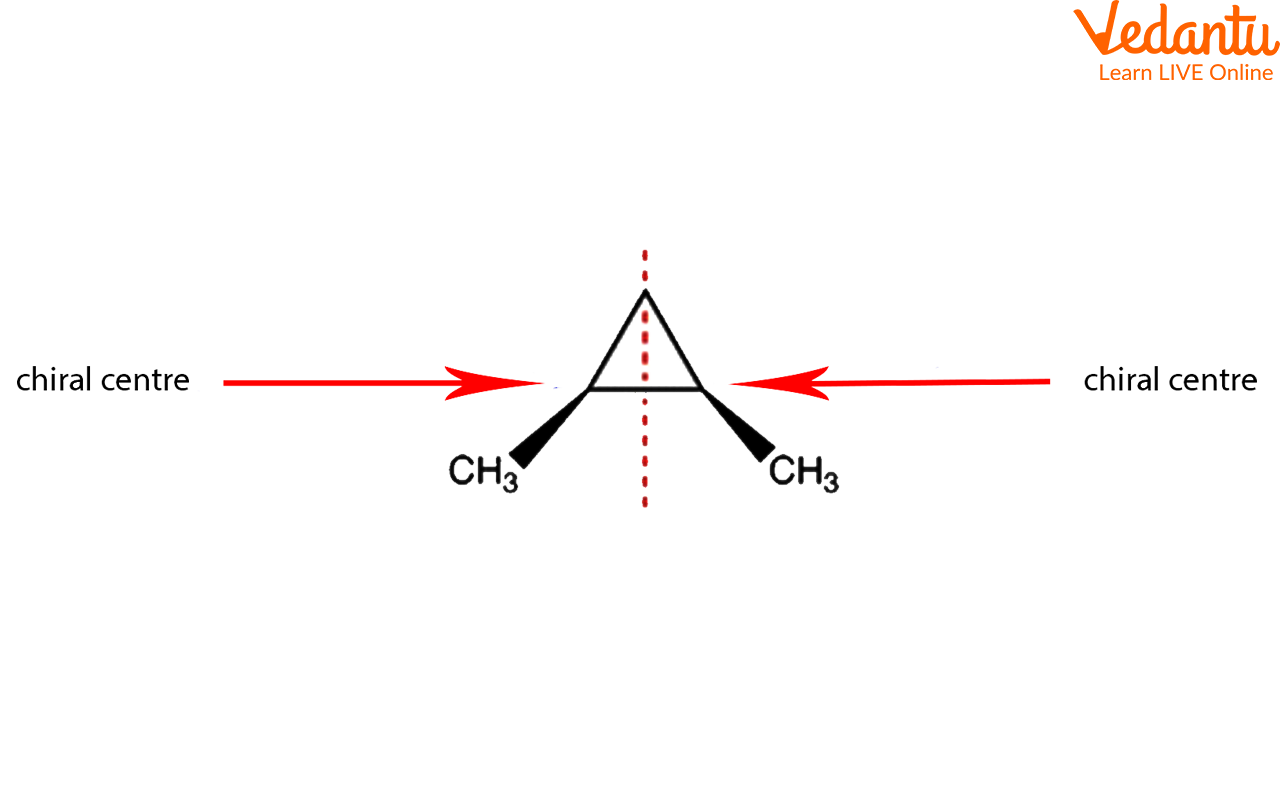
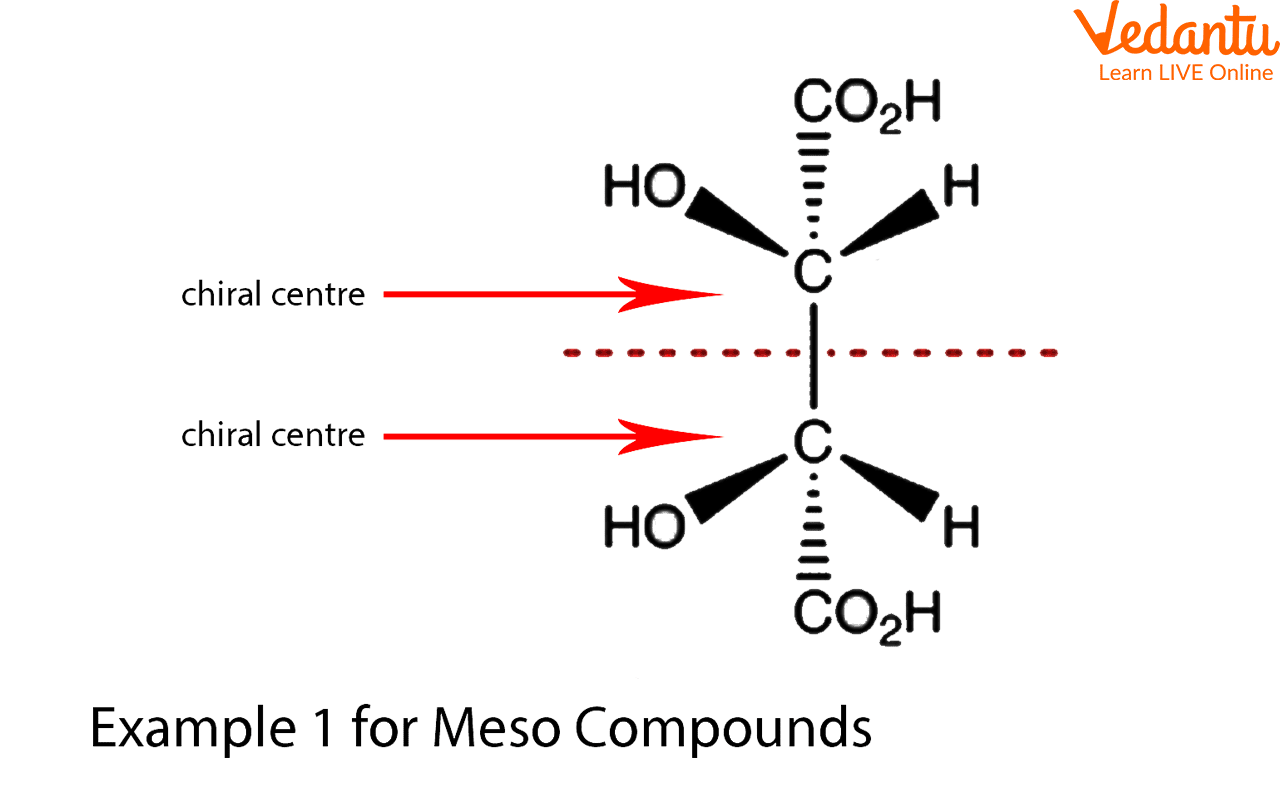
At its core, a meso compound is an achiral molecule that possesses chiral centers. This seemingly paradoxical definition often trips students up. The internal plane of symmetry within the molecule effectively cancels out the chirality arising from the individual chiral centers.
This internal compensation is the key differentiating factor. A molecule must possess at least two chiral centers to even be considered a potential meso compound.
The Importance of Visualizing Symmetry
One of the most significant hurdles students face is accurately visualizing the plane of symmetry. Textbooks often depict molecules in static, two-dimensional forms, which can obscure the three-dimensional reality.
Furthermore, the ability to mentally rotate molecules and identify different conformations is crucial. A conformation that *appears* chiral might, upon rotation, reveal a hidden plane of symmetry.
Molecular modeling kits and software can be invaluable tools. These tools allow students to interact with 3D representations and manipulate molecules, fostering a better understanding of their spatial arrangements.
Common Pitfalls and Misconceptions
Students frequently mistake molecules with chiral centers as inherently chiral themselves. The presence of a chiral center is a *necessary*, but not *sufficient*, condition for chirality.
Another common error lies in misidentifying or overlooking the plane of symmetry. A molecule might have multiple possible conformations, and the plane of symmetry might only be apparent in certain arrangements.
Confusing meso compounds with racemic mixtures is also a prevalent issue. A racemic mixture is an equal mixture of two enantiomers (non-superimposable mirror images), while a meso compound is a single achiral molecule.
Pedagogical Approaches and Challenges
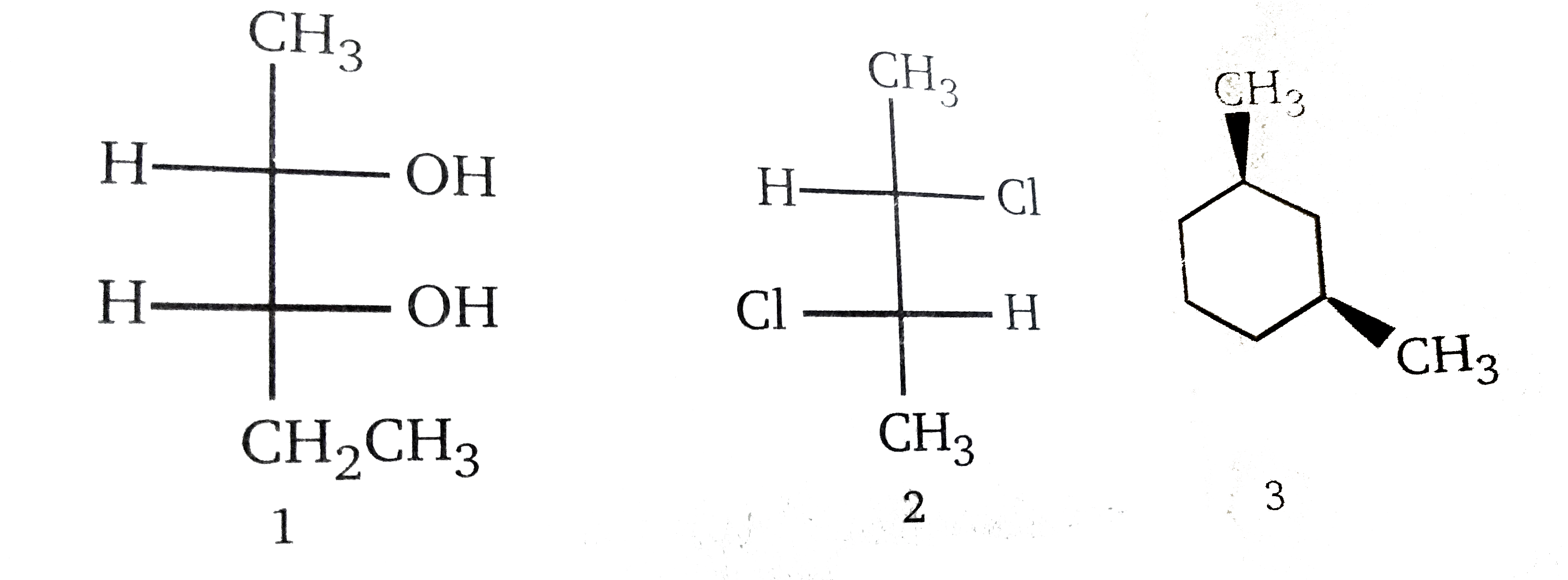
Different instructors utilize varying approaches to teach stereochemistry. Some emphasize the use of wedges and dashes to represent three-dimensional structure, while others rely heavily on Fischer projections.
Each method has its advantages and disadvantages. Wedges and dashes can be more intuitive for some, while Fischer projections can simplify the identification of relationships between stereoisomers. The effectiveness of any approach hinges on the clarity and consistency of the instructor's presentation.
A more hands-on, activity-based approach can also prove effective. Constructing molecular models and engaging in group discussions can facilitate a deeper understanding.
Expert Opinions and Insights
"The concept of meso compounds often serves as a crucial test of a student's understanding of stereochemistry," says Dr. Emily Carter, a chemistry professor at Princeton University. "It requires them to integrate multiple concepts and apply them in a nuanced way."
Dr. Carter also emphasized the importance of early intervention when students struggle. "Addressing misconceptions promptly can prevent them from compounding later on."
Professor David MacMillan, a Nobel laureate in Chemistry, highlights the role of computational chemistry. "Modern software can provide students with interactive visualizations and simulations that were previously unavailable, significantly enhancing their learning experience."
The Future of Stereochemistry Education
The ongoing evolution of technology promises to transform how stereochemistry is taught. Virtual reality (VR) and augmented reality (AR) offer the potential to create immersive learning environments where students can manipulate molecules in a truly three-dimensional space.
The development of interactive online simulations will also play a crucial role. These simulations can provide immediate feedback and allow students to explore different scenarios at their own pace.
Ultimately, a multifaceted approach that combines traditional teaching methods with innovative technologies will be essential. By addressing the underlying challenges and adopting more effective pedagogical strategies, educators can empower students to master the intricacies of stereochemistry and unlock the full potential of this fascinating field.