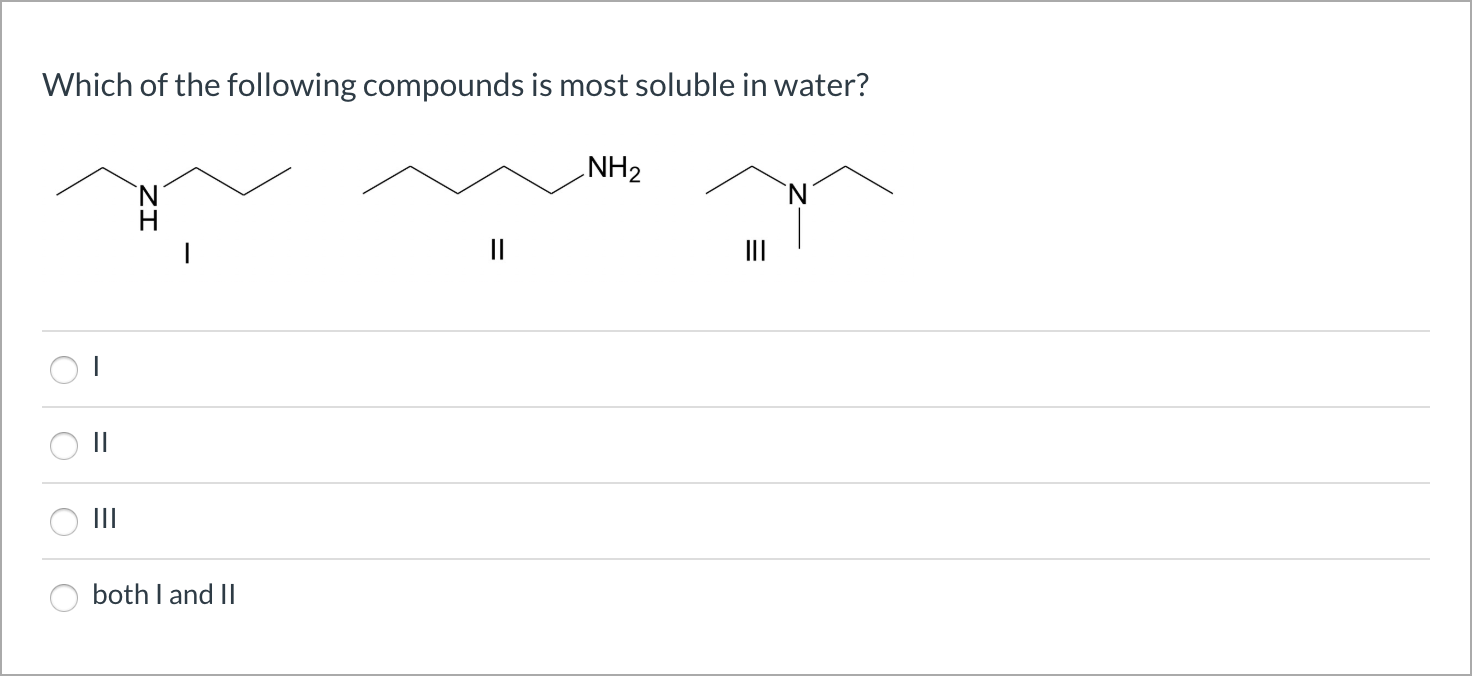
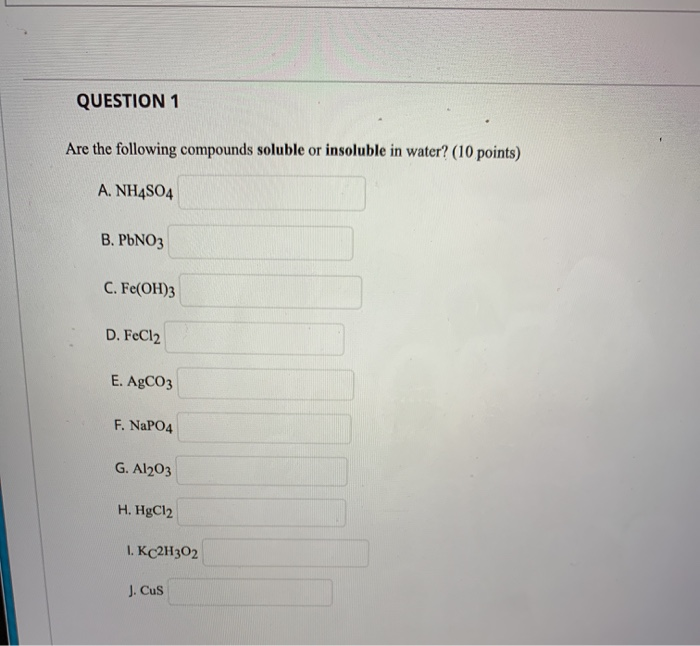
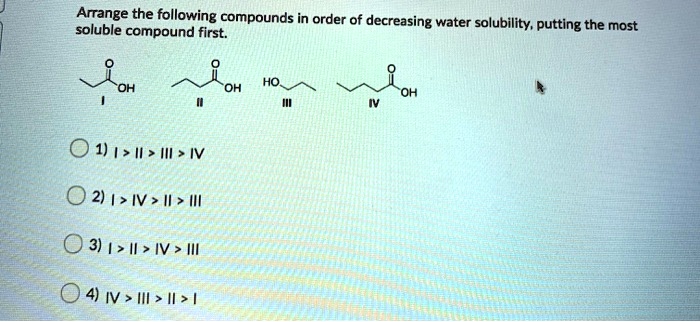
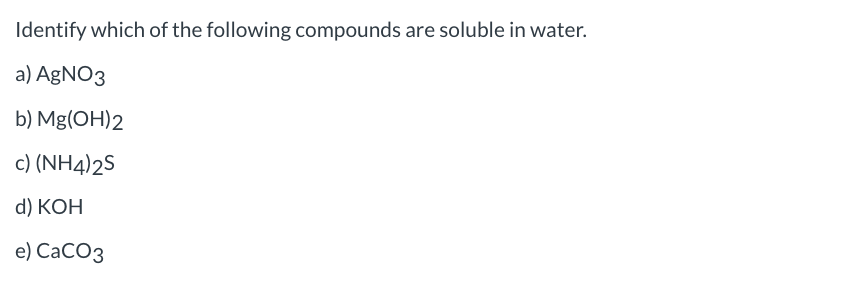Which Of The Following Compounds Is Soluble In Water

The question seems deceptively simple: Which of the following compounds is soluble in water? This fundamental inquiry, often posed in introductory chemistry courses, belies a complex web of intermolecular forces, thermodynamic principles, and real-world implications that ripple through diverse fields, from pharmaceutical development to environmental science. Understanding solubility is not merely an academic exercise; it's a critical skill for manipulating matter at a molecular level.
At its core, the question "Which of the following compounds is soluble in water?" demands a rigorous understanding of how substances interact with water molecules. The answer isn't always intuitive and requires careful consideration of factors like polarity, charge, and the presence of specific functional groups. This article will delve into the intricacies of aqueous solubility, exploring the principles that govern it and highlighting its significance in various scientific disciplines.
The Fundamentals of Aqueous Solubility
Water, often dubbed the "universal solvent," owes its dissolving prowess to its polar nature. The bent molecular structure and electronegativity difference between oxygen and hydrogen create a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. This polarity allows water to form strong electrostatic interactions with other polar or ionic compounds.
The mantra "like dissolves like" summarizes the general rule of solubility. Polar solvents like water tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. This is because the energy required to break the intermolecular forces within both the solvent and the solute must be compensated by the energy released when new interactions form between the solvent and solute molecules.
Ionic Compounds and Solubility
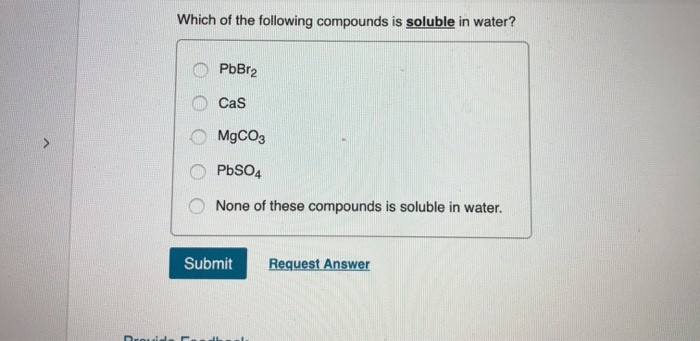
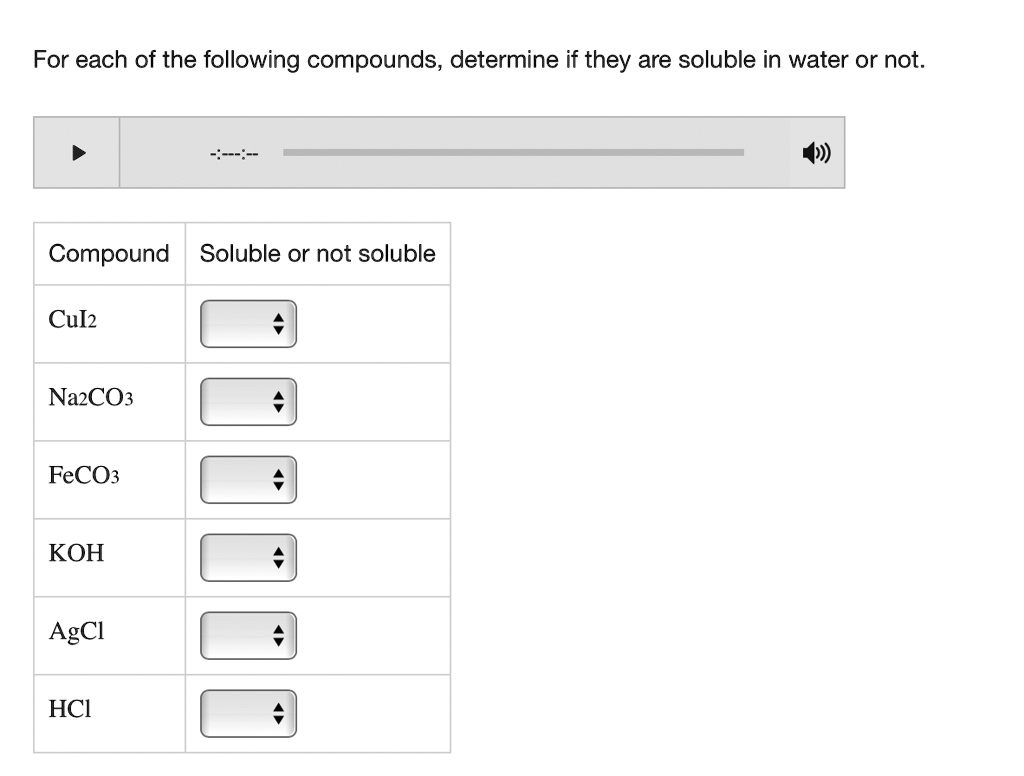
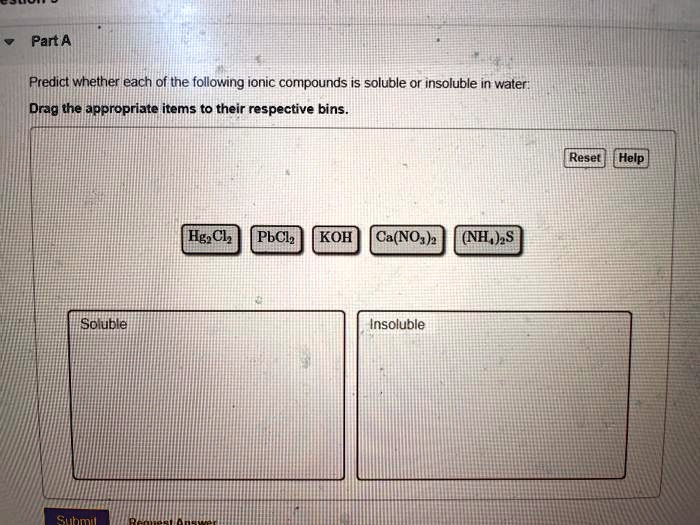
Ionic compounds, composed of positively charged cations and negatively charged anions, can be soluble in water. When an ionic compound dissolves, it dissociates into its constituent ions, which are then surrounded by water molecules. This process, known as hydration, stabilizes the ions in solution.
However, not all ionic compounds are equally soluble. The strength of the electrostatic attraction between the ions in the crystal lattice plays a crucial role. If the lattice energy (the energy required to break apart the crystal lattice) is too high, the hydration energy (the energy released during hydration) may not be sufficient to overcome it, resulting in low solubility.
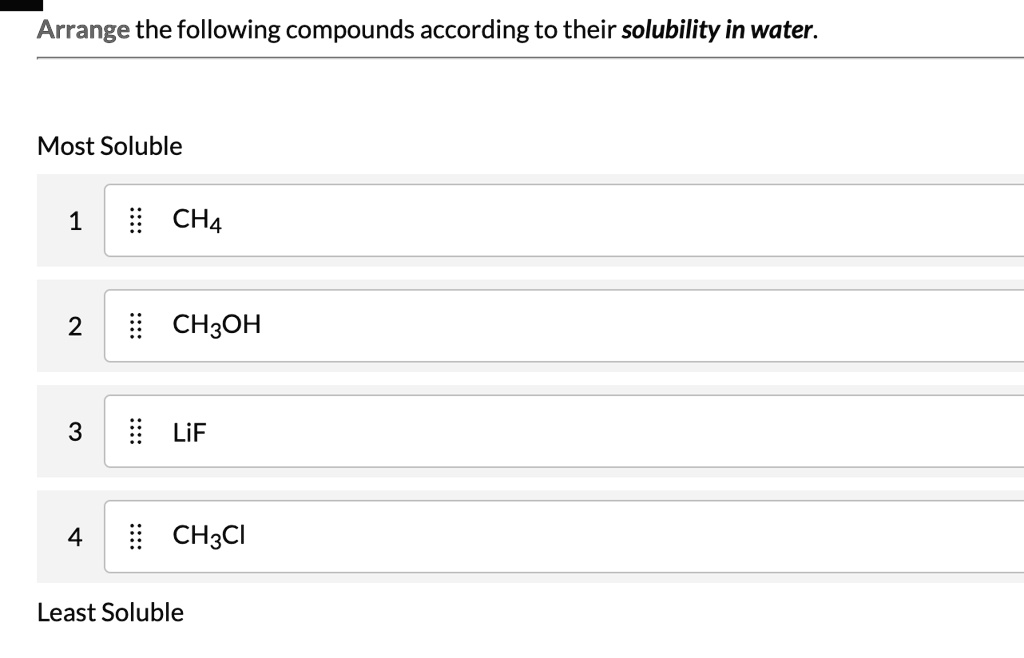
Solubility rules, often presented in introductory chemistry, provide guidelines for predicting the solubility of common ionic compounds. These rules are based on empirical observations and highlight trends in solubility based on the identity of the ions. For example, compounds containing alkali metal ions (Li+, Na+, K+, etc.) and nitrate ions (NO3-) are generally soluble.
Polar Molecular Compounds and Solubility
Polar molecular compounds, unlike ionic compounds, do not dissociate into ions when dissolved in water. Instead, they interact with water molecules through dipole-dipole interactions and hydrogen bonding.
Hydrogen bonding, a particularly strong type of dipole-dipole interaction, occurs when a hydrogen atom is bonded to a highly electronegative atom like oxygen, nitrogen, or fluorine. Water molecules can form hydrogen bonds with other water molecules, as well as with polar molecules containing O-H, N-H, or F-H bonds.
The presence of these functional groups generally increases the solubility of a molecule in water. For example, alcohols (containing the -OH group) and amines (containing the -NH2 group) tend to be more soluble in water than hydrocarbons of comparable size.
Nonpolar Molecular Compounds and Solubility
Nonpolar molecular compounds, such as hydrocarbons (composed of carbon and hydrogen atoms), are generally insoluble in water. These compounds lack significant dipole moments and cannot form strong interactions with water molecules. Instead, they tend to clump together due to London dispersion forces, a type of weak intermolecular attraction.
When a nonpolar compound is mixed with water, the water molecules are forced to rearrange themselves around the nonpolar molecules. This rearrangement reduces the entropy (disorder) of the system, which is thermodynamically unfavorable. As a result, nonpolar compounds tend to separate from water, minimizing the disruption to the water's hydrogen bonding network.
Factors Affecting Solubility
Besides the inherent properties of the solute and solvent, several external factors can influence solubility. Temperature is a key factor; the solubility of most solids in water increases with increasing temperature. This is because higher temperatures provide more kinetic energy, which helps to overcome the lattice energy of the solid.
Pressure, on the other hand, has a significant effect on the solubility of gases in liquids. According to Henry's Law, the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid. This principle explains why carbonated beverages fizz when opened; the decrease in pressure allows the dissolved carbon dioxide to escape from the solution.
The presence of other solutes can also affect solubility. The common ion effect, for example, describes the decrease in solubility of an ionic compound when a soluble salt containing a common ion is added to the solution. This effect is based on Le Chatelier's Principle, which states that a system at equilibrium will shift to relieve stress.
Applications of Solubility
Understanding solubility is crucial in many scientific disciplines. In pharmaceutical science, solubility plays a vital role in drug delivery. A drug must be soluble in bodily fluids to be absorbed into the bloodstream and reach its target site.
In environmental science, solubility affects the fate and transport of pollutants in water. The solubility of contaminants determines how easily they can be dispersed and transported through aquatic ecosystems. Understanding these properties is critical for predicting and mitigating environmental risks.
In chemical synthesis, solubility is essential for selecting appropriate solvents for reactions. A suitable solvent must be able to dissolve the reactants and products, facilitate the reaction, and be easily removed after the reaction is complete. The solubility of materials is also very important when dealing with materials to be used in the food and beverage industry.
Conclusion
The seemingly simple question of which compounds are soluble in water reveals a complex interplay of intermolecular forces, thermodynamic principles, and practical applications. By understanding the factors that govern solubility, we can better predict and control the behavior of matter in aqueous solutions, leading to advancements in diverse fields. Further research on how newly emerging pollutants of concern react in different ecosystems, may allow for the discovery of new ways to protect human health and the environment.