Which Of The Following Factors Influence Molecular Speed
Molecular speed is not a constant. Temperature and molecular mass are the primary factors dictating how quickly molecules move.
Understanding the determinants of molecular speed is crucial for various scientific disciplines, from chemistry to atmospheric science. This knowledge impacts how we predict reaction rates, model gas behavior, and comprehend various physical phenomena.
Key Factors Influencing Molecular Speed
Temperature: The Primary Driver
Temperature is the most direct influence on molecular speed. Higher temperatures mean molecules have more kinetic energy, resulting in faster movement.
The relationship is proportional: as temperature increases, the average speed of molecules increases as well. This is described quantitatively by the kinetic molecular theory.
Specifically, the root-mean-square speed (vrms) of a molecule is directly proportional to the square root of the absolute temperature (in Kelvin).
vrms = √(3RT/M)where R is the ideal gas constant and M is the molar mass.
Molecular Mass: The Counterbalance
Molecular mass has an inverse relationship with molecular speed. Heavier molecules move slower than lighter molecules at the same temperature.
This is because kinetic energy is dependent on both mass and velocity. For a given kinetic energy (determined by temperature), a larger mass necessitates a smaller velocity.
Again, referring to the equation, vrms = √(3RT/M), one can easily observe that vrms decreases as M (molar mass) increases, assuming temperature (T) remains constant. A heavier molecule must, therefore, travel slower at the same temperature.
Other Considerations
While temperature and molecular mass are the dominant factors, other variables can play a role in specific scenarios. Pressure and intermolecular forces, while less direct, can influence molecular motion.
Pressure indirectly affects molecular speed by influencing the frequency of collisions. Higher pressure leads to more frequent collisions, which can alter the direction and, to some extent, the overall movement of molecules, though it does not directly alter the speed dictated by temperature and mass.
Intermolecular forces, such as van der Waals forces or hydrogen bonding, can also influence molecular speed, especially in liquids and dense gases. Stronger intermolecular forces can slow down molecular movement as molecules are more attracted to each other.
Data and Evidence
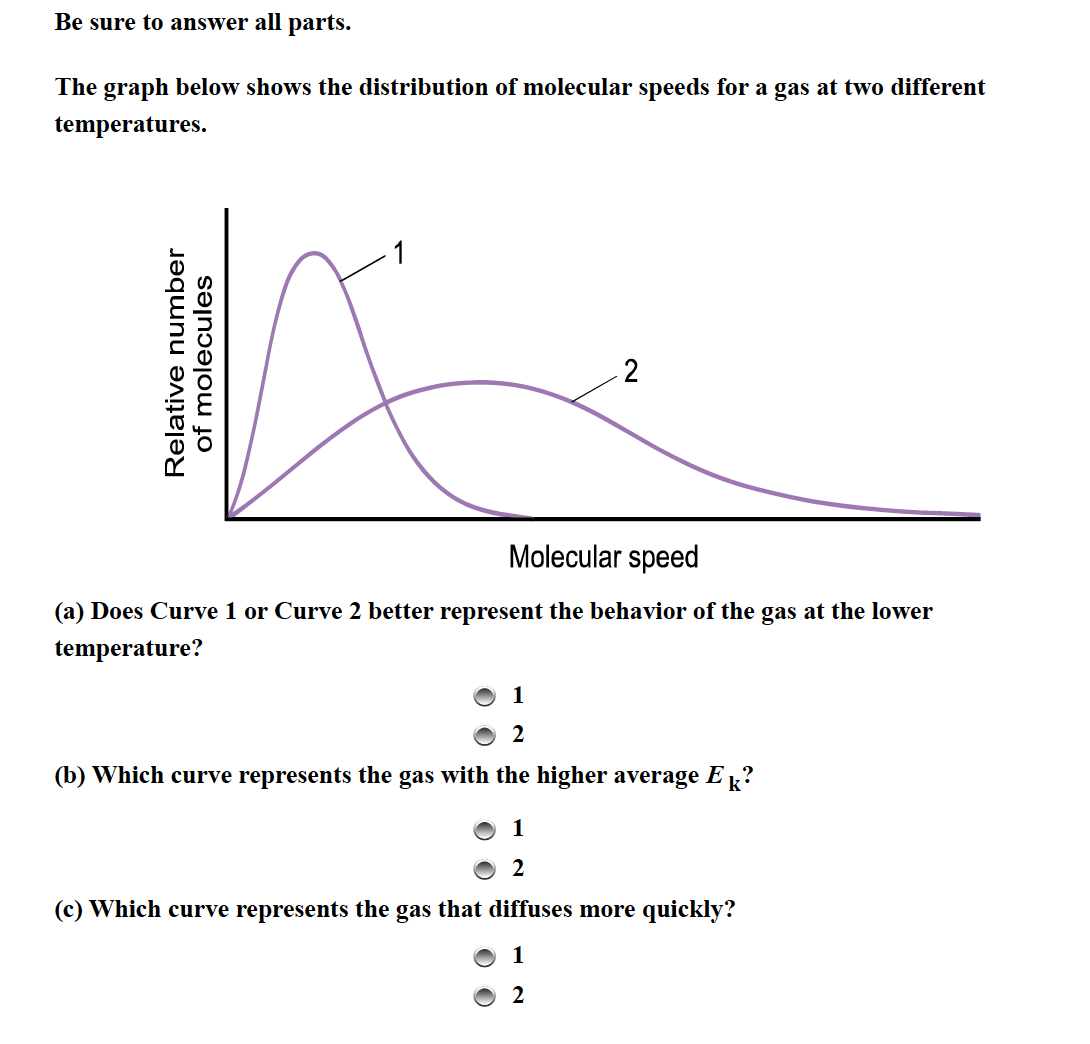
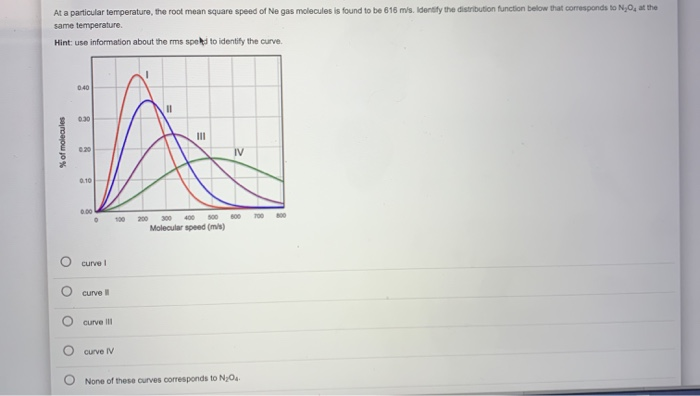
Numerous experiments and simulations validate the relationship between temperature, molecular mass, and molecular speed. The Maxwell-Boltzmann distribution, a statistical distribution, describes the speeds of molecules in a gas at a given temperature.
This distribution confirms that at higher temperatures, the curve shifts to the right, indicating higher average speeds. Similarly, gases with heavier molecules exhibit distributions shifted to the left, indicating lower average speeds at the same temperature.
Data from spectroscopic techniques, such as Doppler broadening measurements, also directly correlate temperature and molecular speed. The Doppler broadening of spectral lines is directly proportional to the average speed of the molecules.
Implications and Applications
Understanding the factors influencing molecular speed is essential in fields like chemical kinetics. The rate of a chemical reaction is influenced by the speed at which molecules collide.
In atmospheric science, this knowledge helps predict the movement and dispersion of pollutants. Lighter pollutants will disperse faster than heavier ones, and temperature plays a key role in dispersal rates.
Additionally, this understanding is crucial in designing and optimizing industrial processes that involve gases or liquids. Controlling temperature and pressure can significantly affect reaction yields and separation efficiencies.
Next Steps
Ongoing research is focused on understanding how these factors interact in complex systems. Scientists are exploring the behavior of molecules in extreme conditions, such as high pressures and temperatures.
Further investigation into the effects of intermolecular forces on molecular speed in non-ideal gases and liquids is also crucial. This will provide a more complete picture of molecular motion in real-world scenarios.
Advanced computational models are being developed to simulate molecular behavior with increased accuracy. These models will help predict and optimize various processes, from drug delivery to material design.