Is Glucose Oxidized Or Reduced In Cellular Respiration
+is+oxidized%2C+and+O2+is+reduced:+becomes+oxidized..jpg)
Imagine a tiny spark, a minuscule explosion of energy occurring within the silent chambers of every cell in your body. This isn't some futuristic fantasy; it's the fundamental process of cellular respiration, the engine that fuels life itself. At the heart of this process lies a seemingly simple question, yet one with profound implications: what happens to glucose?
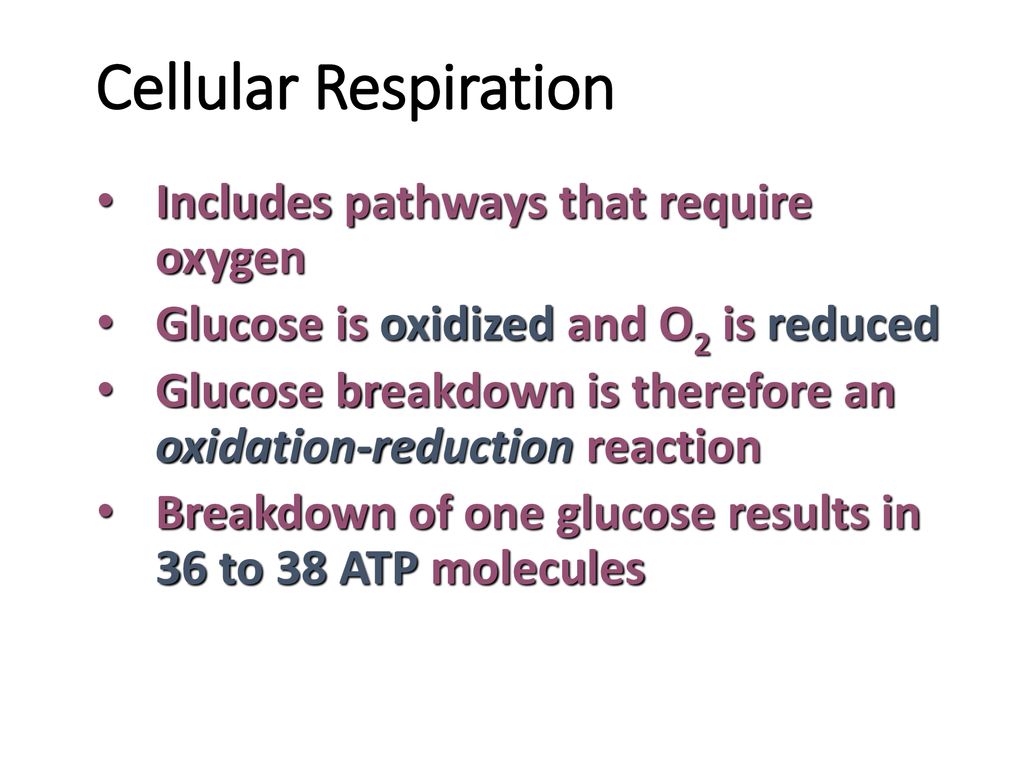
The central question we'll unravel is whether glucose undergoes oxidation or reduction during cellular respiration. The answer, unequivocally, is oxidation. Understanding this clarifies how our bodies extract energy from the food we eat and highlights the elegant dance of electrons that sustains us.
The Dance of Electrons: Oxidation and Reduction
To understand why glucose is oxidized, we need to revisit the fundamental concepts of oxidation and reduction. These aren't just abstract chemistry terms; they represent the transfer of electrons, the tiny particles that carry energy within atoms.
Oxidation, in its simplest form, is the loss of electrons. Think of it as a substance giving away some of its energy carriers. Conversely, reduction is the gain of electrons, a substance accepting those energy carriers and becoming more energy-rich.
These two processes always occur together, hence the term redox reaction. One substance is oxidized (loses electrons), and another is reduced (gains electrons).
Cellular Respiration: A Step-by-Step Unveiling
Cellular respiration is the process by which cells break down glucose to release energy in the form of ATP (adenosine triphosphate), the cell's energy currency. It's a complex, multi-stage process, but we can broadly divide it into three main stages: glycolysis, the Krebs cycle (also known as the citric acid cycle), and the electron transport chain.
Glycolysis: The Initial Breakdown
Glycolysis occurs in the cytoplasm of the cell. Glucose, a six-carbon sugar, is broken down into two molecules of pyruvate, a three-carbon molecule.
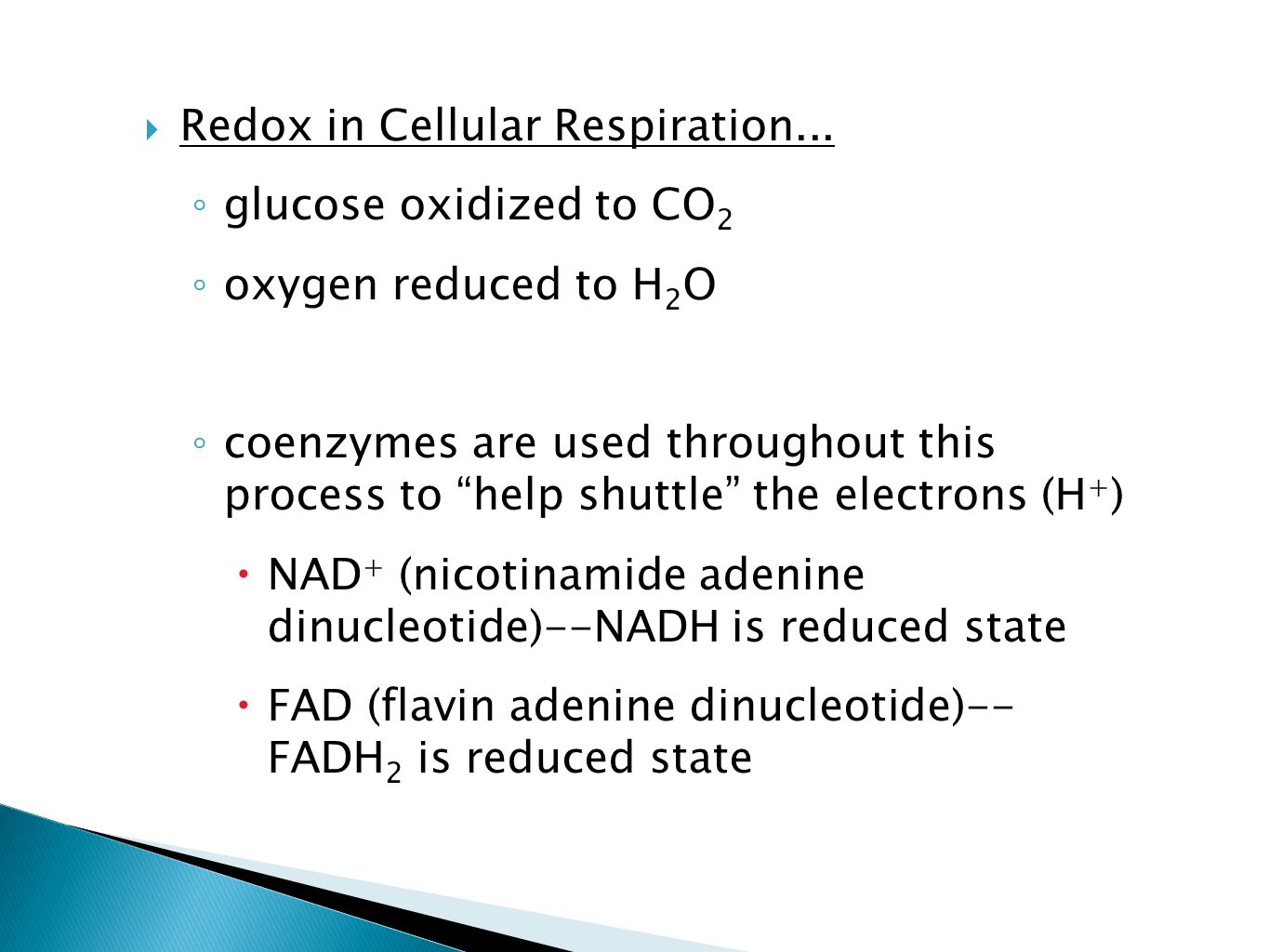
During this process, some ATP is produced directly, and some NAD+ (nicotinamide adenine dinucleotide) is reduced to NADH. This NADH carries electrons to the later stages of respiration.
The Krebs Cycle: Releasing More Electrons
Pyruvate is transported into the mitochondria, the powerhouse of the cell, where it is converted into acetyl-CoA. Acetyl-CoA then enters the Krebs cycle.
In the Krebs cycle, a series of reactions further oxidize the acetyl-CoA, releasing carbon dioxide (CO2) as a waste product. More ATP is produced, but importantly, more NAD+ and FAD (flavin adenine dinucleotide) are reduced to NADH and FADH2, respectively.
These reduced electron carriers are now brimming with energy, ready to deliver it to the final stage.
The Electron Transport Chain: Harvesting the Energy
The electron transport chain (ETC) is located in the inner mitochondrial membrane. NADH and FADH2 deliver their electrons to a series of protein complexes.
As electrons move down the chain, energy is released. This energy is used to pump protons (H+) across the membrane, creating a concentration gradient.
The protons then flow back across the membrane through ATP synthase, an enzyme that uses the energy of this flow to produce large amounts of ATP.
Finally, the electrons and protons combine with oxygen to form water (H2O), which is another waste product.
Why Glucose is Oxidized: The Evidence
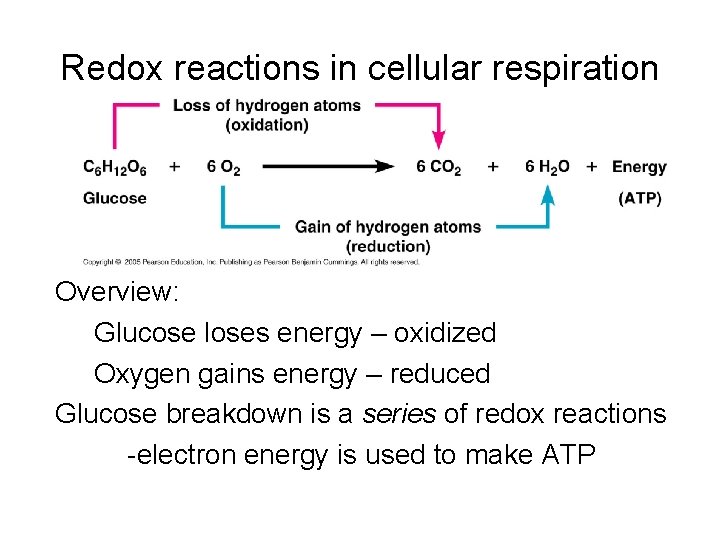
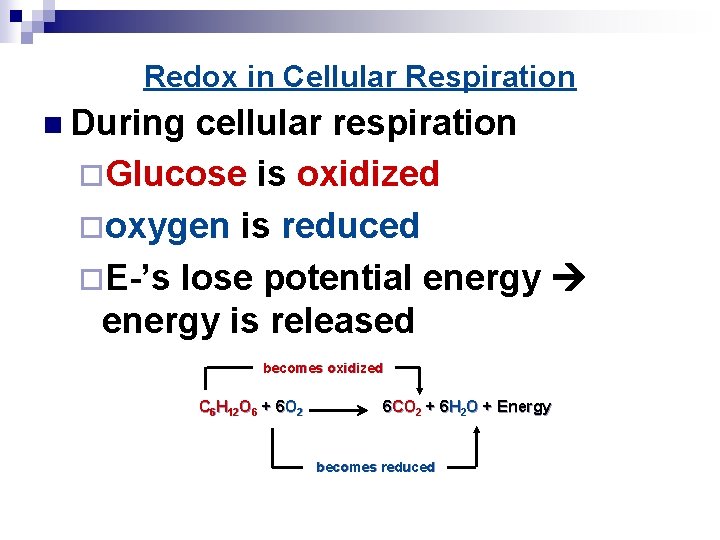
Throughout cellular respiration, glucose is systematically broken down, its carbon atoms gradually losing electrons. These electrons are picked up by electron carriers like NAD+ and FAD, which are reduced.
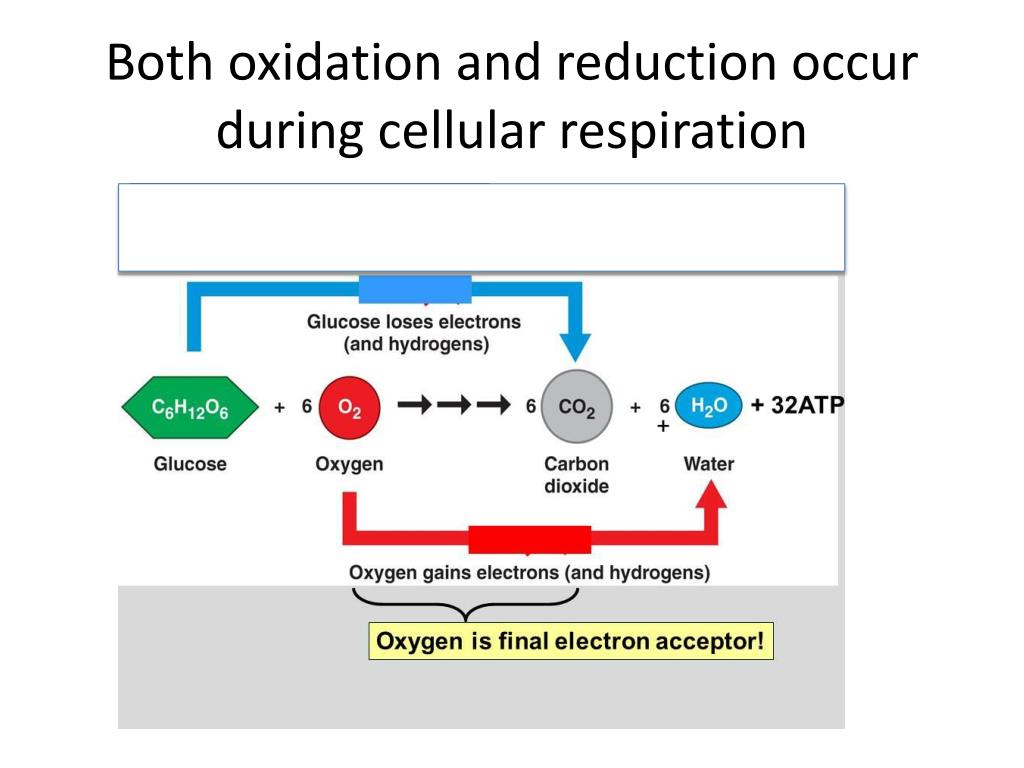
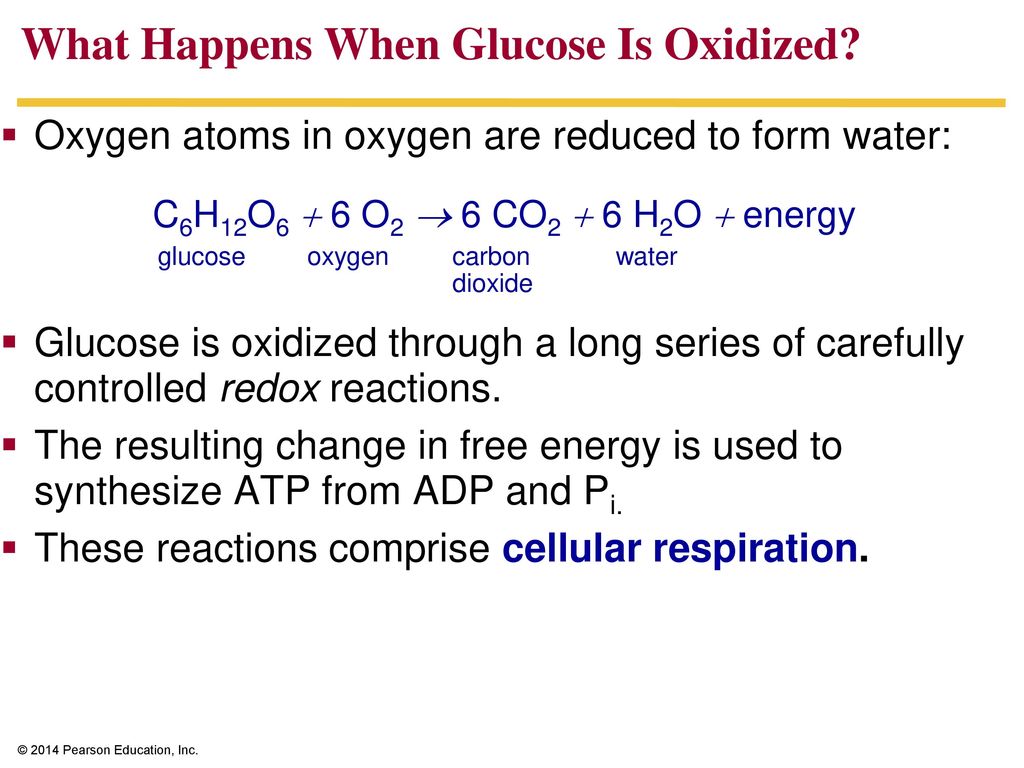
The end products of glucose oxidation are carbon dioxide and water. Carbon dioxide is a low-energy molecule, indicating that the carbon atoms have lost much of their energy-holding electrons.
Water also contains oxygen atoms that have been reduced, accepting electrons from the electron transport chain. This directly supports the initial claim that glucose is oxidized.
Consider the overall chemical equation for cellular respiration: C6H12O6 + 6O2 → 6CO2 + 6H2O. Observe how the carbon in glucose (C6H12O6) ends up in carbon dioxide (6CO2). The carbon has gained oxygen atoms, but more importantly, it has lost electrons (shared with the oxygen atoms), signifying oxidation.
The Significance of Glucose Oxidation
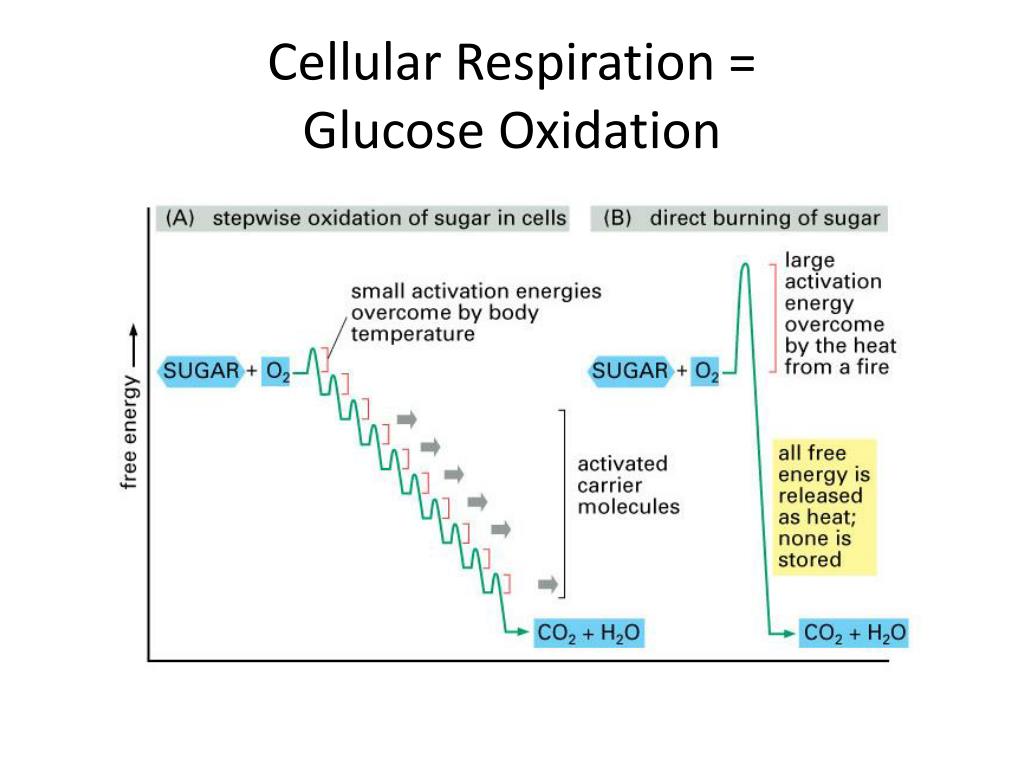
The fact that glucose is oxidized, not reduced, is fundamental to how we obtain energy from food. The stepwise oxidation of glucose allows for a controlled release of energy.
If glucose were to be oxidized all at once, the energy would be released as heat, which would damage the cell. By carefully controlling the transfer of electrons, cellular respiration can efficiently capture the energy and store it in the form of ATP.
This process is essential for all living organisms that rely on cellular respiration for energy. From the smallest bacteria to the largest whales, the oxidation of glucose is the engine that drives life.
Beyond the Textbook: Implications for Health
Understanding glucose oxidation has practical implications for health. Conditions like diabetes involve disruptions in glucose metabolism, leading to impaired energy production.
Type 2 diabetes, for example, is characterized by insulin resistance, meaning that cells are less responsive to insulin, a hormone that helps glucose enter cells. This can lead to elevated blood glucose levels and impaired cellular respiration.
Understanding the mechanisms of glucose oxidation and the factors that regulate it is crucial for developing effective treatments for metabolic disorders.
A Reflective Conclusion
The seemingly simple question of whether glucose is oxidized or reduced in cellular respiration unlocks a deeper understanding of the intricate processes that sustain life. It’s not just about memorizing facts; it’s about appreciating the elegance and efficiency of biological systems.
Next time you eat a meal, remember the tiny sparks of energy igniting within your cells, the oxidation of glucose fueling your every breath, every movement, every thought. It's a testament to the remarkable chemistry that makes life possible.






+and+oxygen+is+reduced+to+form+water..jpg)