Which Of The Following Is Not A Property Of Metals

Imagine holding a gleaming copper wire, its surface catching the light like a miniature sunset. Feel its weight, its solidity, a tangible promise of conducting electricity to power your home. Now, consider a brittle piece of chalk, easily snapped in two. The contrast is stark, a fundamental difference in the nature of these materials, leading us to the question: which characteristic doesn't belong in the realm of metals?
This article delves into the fascinating world of metals, exploring their defining properties like conductivity, malleability, ductility, luster, and strength. We'll examine why one common misconception about metals stands apart, a trait that sets them distinctly apart from the norm, revealing the nuanced nature of these essential elements.
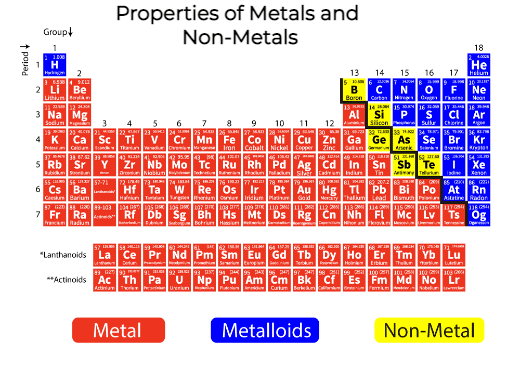
Unveiling the Metallic Identity
Metals have been instrumental in shaping civilization, from the Bronze Age to the Information Age. Their unique properties have allowed us to build infrastructure, create advanced technologies, and even craft beautiful works of art. Understanding these properties is crucial to appreciating their role in our world.
So, what makes a metal a metal? What shared characteristics bind gold, iron, and aluminum together?
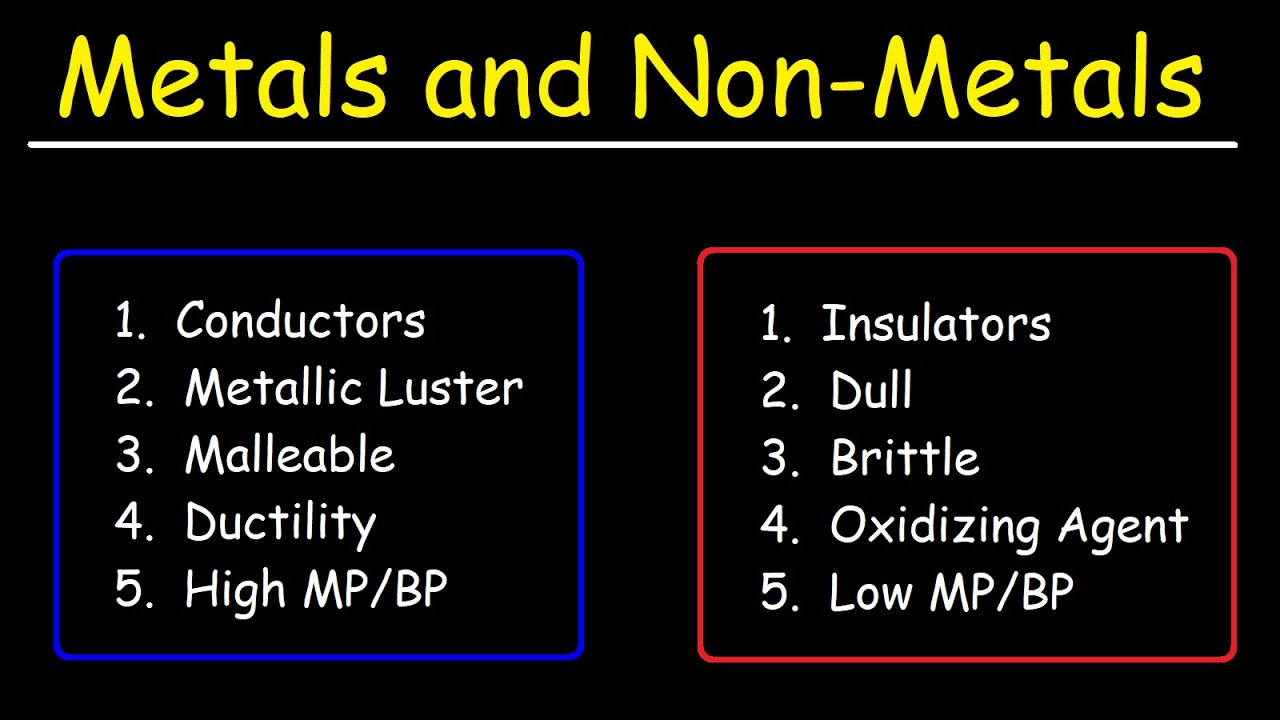
The Usual Suspects: Metallic Properties
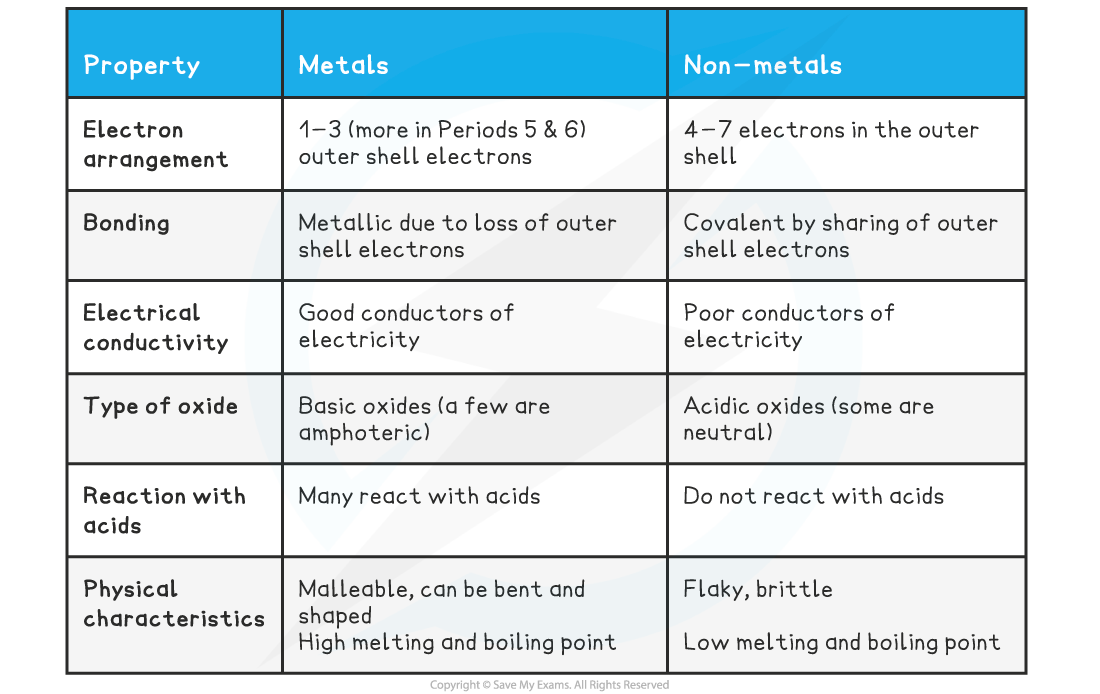
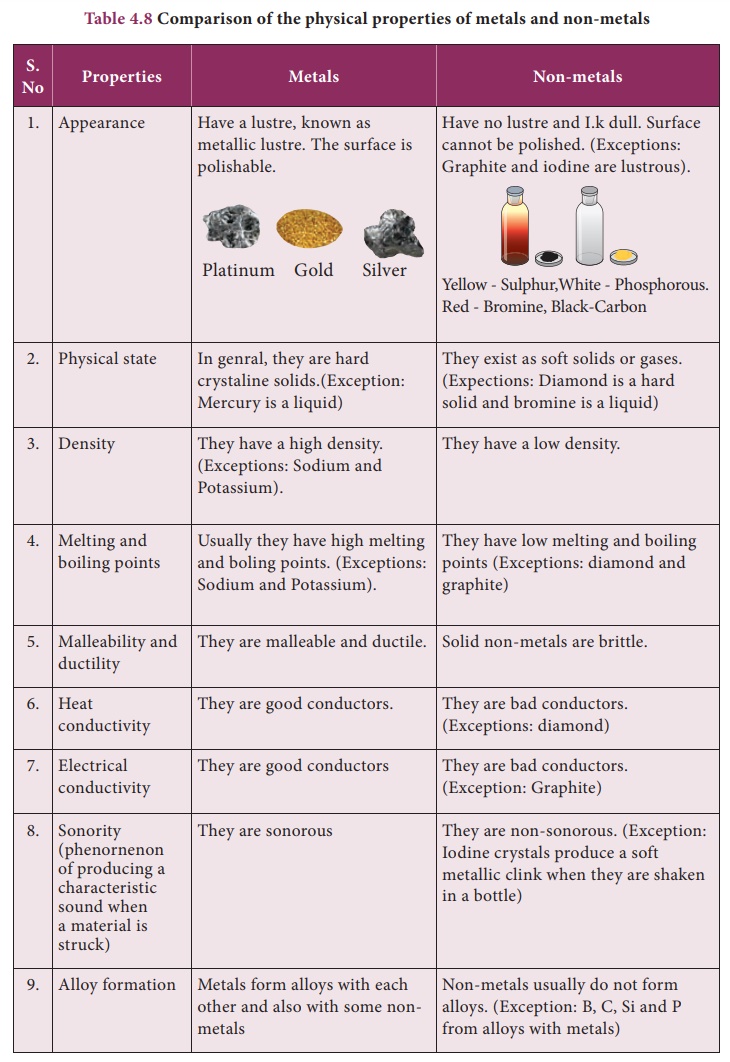
One of the most defining characteristics of metals is their excellent conductivity. They are superb conductors of both heat and electricity, making them indispensable in electrical wiring and heating elements.
This remarkable ability stems from the "sea of electrons" model, where valence electrons are delocalized and free to move throughout the metallic lattice. This freedom allows electrons to easily transport energy, explaining both electrical and thermal conductivity.
Another key property is malleability, the ability to be hammered or rolled into thin sheets without breaking. Think of the delicate gold leaf used in decorative applications or the aluminum foil in your kitchen.
Closely related to malleability is ductility, the capacity to be drawn into wires. Copper, for instance, is widely used in electrical wiring precisely because of its ductility.
Metals also possess a characteristic luster, a shiny or reflective surface. This is due to the interaction of light with the free electrons on the metal's surface.
Finally, most metals are known for their strength and tensile strength, meaning they can withstand significant force without deforming or breaking. Steel, an alloy of iron and carbon, is a prime example of a strong metal used in construction.
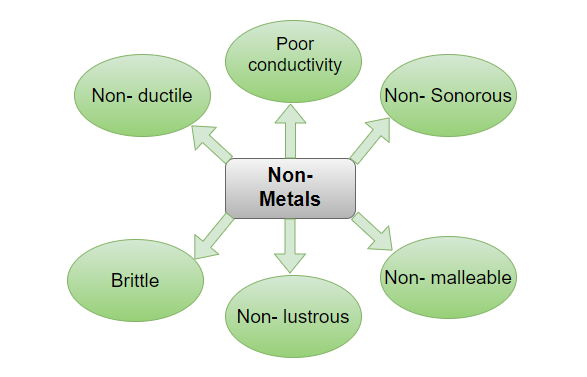
The Exception to the Rule: Brittleness
While the aforementioned properties are commonly associated with metals, one trait is distinctly not a hallmark of their identity: brittleness. Brittleness is the tendency to fracture or break easily when subjected to stress.
While some metals can exhibit brittle behavior under certain conditions, it's not an inherent property like conductivity or malleability. In general, metals are known for their ability to deform before fracturing, a characteristic opposite of brittleness.
Therefore, if asked "Which of the following is not a property of metals?", brittleness would be the correct answer.
Context Matters: When Metals Behave Differently
It's important to note that the properties of metals can be influenced by factors like temperature, impurities, and the presence of alloys. For instance, some metals become brittle at very low temperatures.
The addition of certain elements to create alloys can also alter a metal's properties. For example, adding carbon to iron creates steel, which is significantly stronger than pure iron.
Even the manufacturing process can affect a metal's characteristics. Processes like cold working can increase strength but also reduce ductility, potentially making the metal more prone to brittle fracture under certain conditions.
Beyond the Textbook: Practical Applications
Understanding the properties of metals has immense practical implications. Engineers carefully select materials based on their specific properties when designing structures, machines, and electronic devices.
The high conductivity of copper makes it ideal for electrical wiring, while the strength and ductility of steel make it suitable for bridges and buildings. The resistance of some metals to corrosion makes them suitable for containers in contact with strong chemicals or for building ships.
The malleability of gold allows jewelers to create intricate designs, and the high melting point of tungsten makes it perfect for light bulb filaments.
A Deeper Reflection on Metallic Nature
The journey into the world of metals reveals not just a set of physical properties, but also the ingenuity of human innovation. Metals, with their unique combination of characteristics, have been harnessed to solve complex problems and enrich our lives in countless ways.
Recognizing that brittleness is not a typical property of metals reinforces our understanding of their fundamental nature. This knowledge empowers us to make informed choices about materials and appreciate the role metals play in shaping our world.
From the humblest nail to the most sophisticated microchip, metals continue to drive technological advancements and define the modern landscape. It’s a testament to the power of understanding the fundamental properties of the materials around us.









.png)