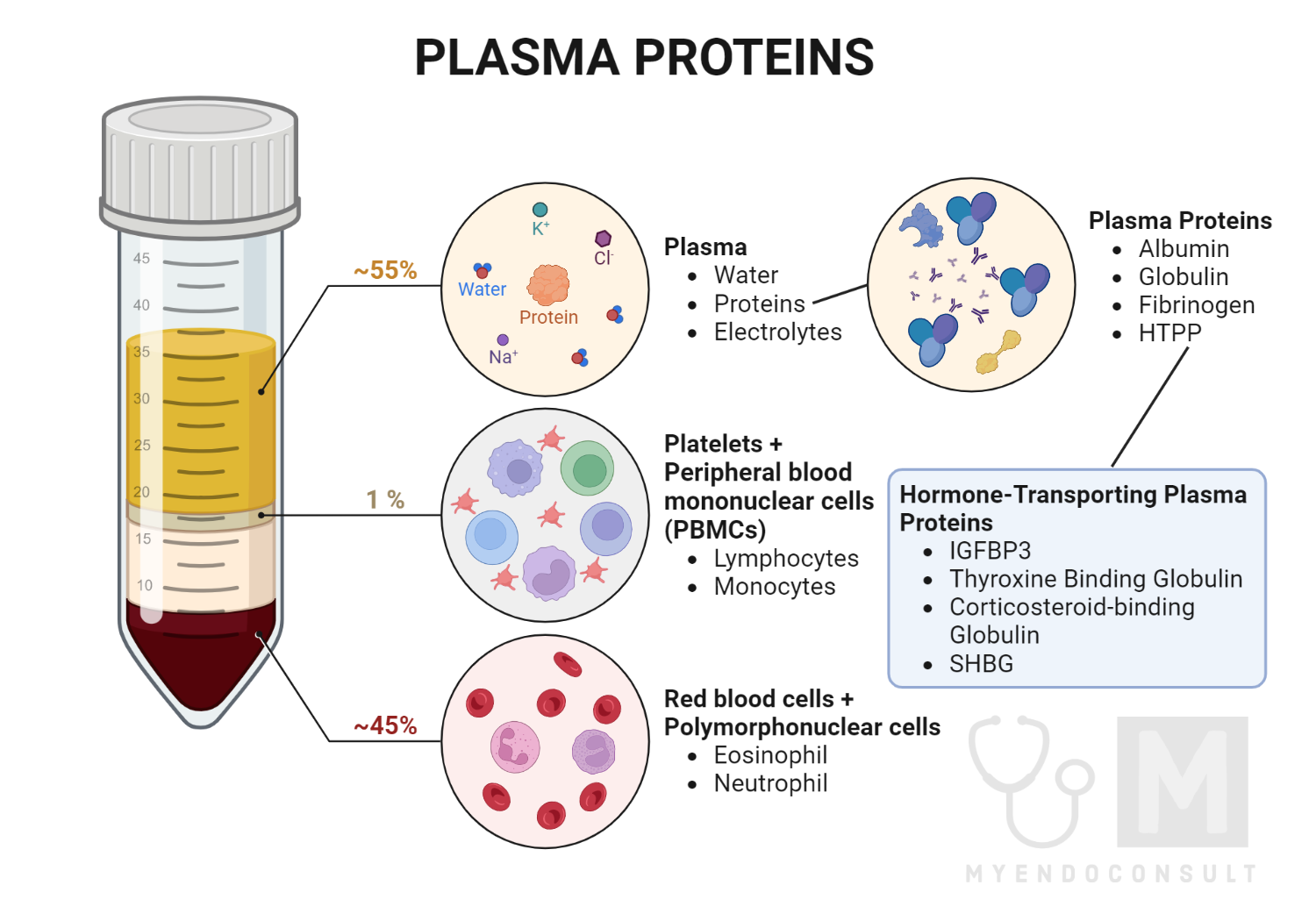
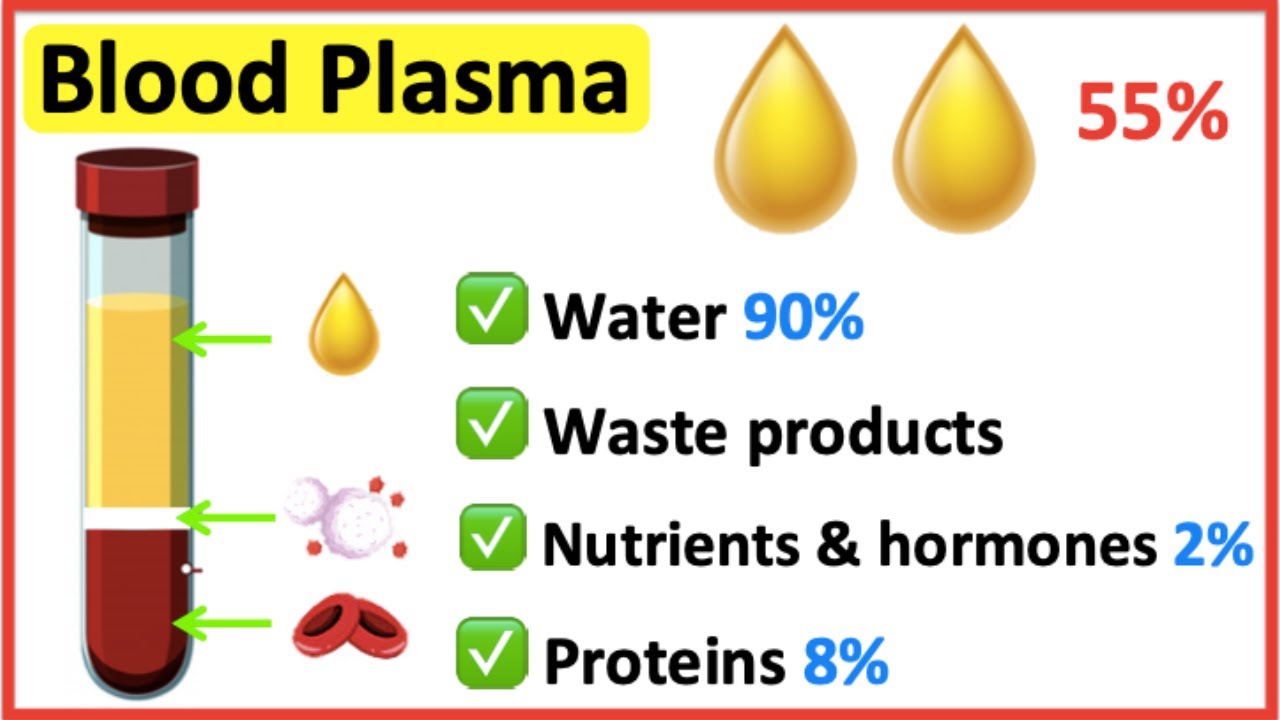
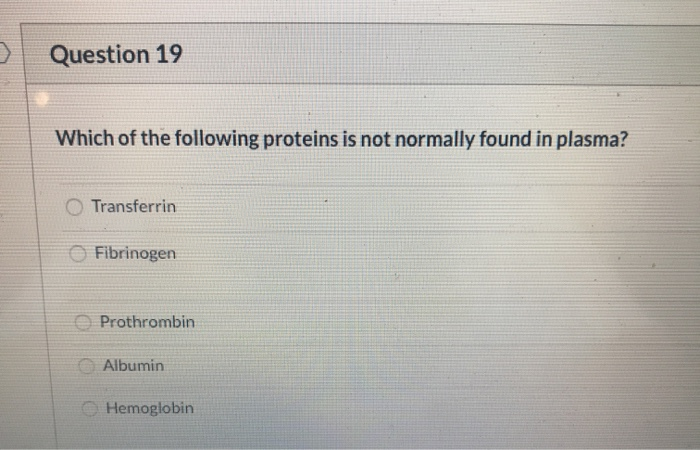
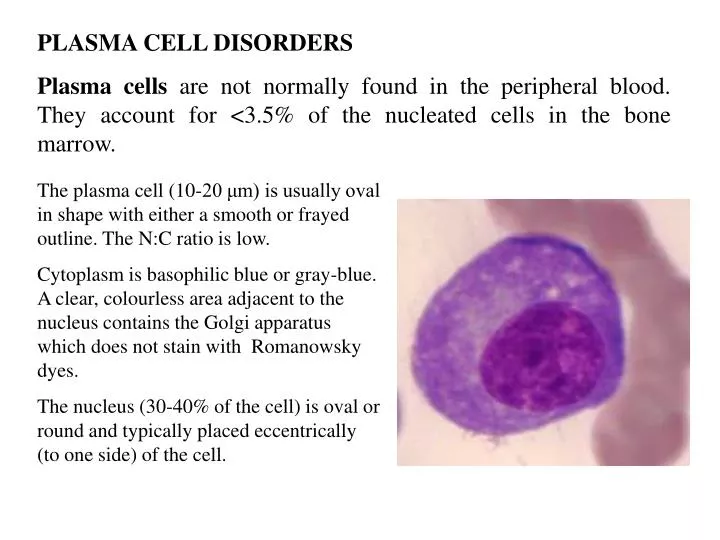
Which Of The Following Is Not Normally Found In Plasma
Imagine a lightning storm, the raw energy crackling across the sky. Or picture the mesmerizing glow of a neon sign, a vibrant dance of light. These seemingly disparate phenomena share a common thread: plasma, the fourth state of matter. But what exactly isn't found in this energetic soup of particles?
The question "Which of the following is not normally found in plasma?" seems simple, yet it unveils a fascinating realm of physics. The answer, broadly speaking, is neutral atoms in their ground state. Understanding why requires a journey into the heart of plasma and its unique properties.
Plasma, often called the "fourth state of matter," is essentially a gas that has become so energized that its atoms have lost some or all of their electrons. This process, known as ionization, results in a mixture of ions (atoms with a net positive charge) and free electrons.
The Formation and Characteristics of Plasma
To understand what's not in plasma, we first need to understand what is. Plasma is created when a gas is heated to extremely high temperatures or subjected to a strong electromagnetic field.
This intense energy strips electrons from the atoms, forming a collection of positively charged ions and negatively charged electrons. This collection can be described as a sea of charged particles.
This mixture behaves unlike a normal gas. It conducts electricity, interacts strongly with magnetic fields, and emits electromagnetic radiation (light, radio waves, etc.).
Examples of plasma are everywhere. The sun and other stars are largely composed of plasma. Lightning is a fleeting example, as is the Earth's ionosphere and even the gas inside fluorescent light bulbs.
Neutral Atoms: The Uncommon Guest
While plasma primarily consists of ions and free electrons, some neutral atoms may still be present. However, these neutral atoms are typically in an excited state, not their ground state.
An atom in its ground state has its electrons in the lowest possible energy levels. But, the intense energy environment of plasma excites the electrons in the neutral atoms to higher energy levels.
These excited atoms quickly release this energy in the form of photons (light), returning to a lower energy state, but not necessarily the ground state. A significant population of atoms in the ground state would indicate the gas is not fully ionized, therefore, it isn't fully plasma.
Degrees of Ionization
It's important to note that the degree of ionization in a plasma can vary widely. Some plasmas, like those in the core of the sun, are almost fully ionized.
Meaning practically all atoms have lost all their electrons. Other plasmas, such as those used in industrial applications, may only be partially ionized.
In partially ionized plasmas, a significant fraction of neutral atoms can exist. Still, those atoms are likely to be in excited state.
Even in these cases, though, the distinguishing characteristic of plasma is the presence and dominance of ions and free electrons, dictating its behavior and properties.
Plasma's Impact on Technology and Beyond
Plasma's unique properties have made it invaluable in numerous technological applications. From fusion energy research to semiconductor manufacturing, plasma plays a critical role.
In medicine, plasma is used for sterilization and wound healing. In industry, it's used for surface treatment and etching.
The study of plasma is also crucial for understanding astrophysical phenomena, such as solar flares and auroras. As technology advances, the understanding of plasma will continue to be a fundamental and interdisciplinary field of study.
The ongoing research on plasma physics is providing us with innovative technological advancements, in industries from semiconductors to energy.
Reflections on the Energetic World of Plasma
The question of what's not typically found in plasma – neutral atoms in their ground state – opens a window into the complex and fascinating world of ionized gases. It highlights the energetic nature of plasma, where atoms are stripped of their electrons, creating a sea of charged particles that governs its behavior.
From the shimmering glow of a neon sign to the immense power of the sun, plasma surrounds us, driving technological innovation and shaping our understanding of the universe. So, while neutral atoms might occasionally be present, the essence of plasma lies in its charged constituents, a dynamic and energetic state of matter unlike any other.
As we continue to explore the potential of plasma, we are sure to unlock even more profound insights into the nature of matter and its transformative applications for the future.


![Which Of The Following Is Not Normally Found In Plasma [Solved]: QUESTION 6 Refer to the figure. Which of the follo](https://media.cheggcdn.com/study/59c/59caa71e-d773-48e0-8ce1-be3b1f817039/image.jpg)