Which Of The Following Is True Regarding Herbal Supplements
Urgent health alert: The safety and efficacy of herbal supplements remain largely unverified, leaving consumers vulnerable to potential risks. Misleading marketing and inconsistent regulation contribute to widespread confusion and potential harm.
This article cuts through the noise, delivering critical facts about herbal supplements. Understand the truth and protect your health with this essential guide.
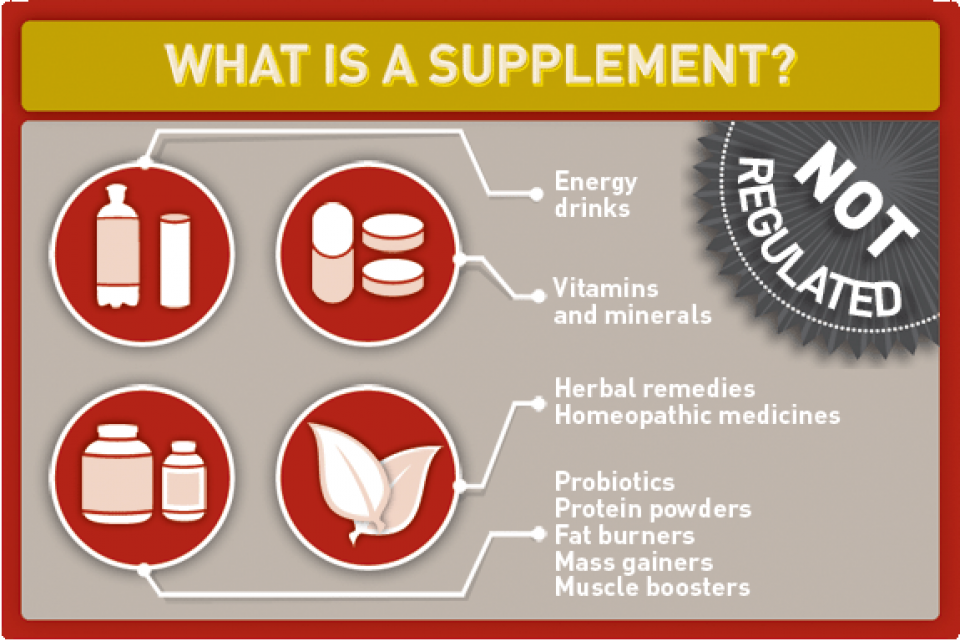
Lack of FDA Regulation: A Critical Gap
The Food and Drug Administration (FDA) regulates herbal supplements as food, not drugs.
This means supplements do not undergo the rigorous testing and approval processes required for pharmaceuticals, creating a significant safety gap.
According to the National Institutes of Health (NIH), manufacturers are not required to prove the safety or effectiveness of their products before they are sold.
Inconsistent Manufacturing Practices: A Recipe for Risk
Manufacturing standards for herbal supplements are often inconsistent.
A 2015 study published in the journal BMC Medicine found that many supplements contained ingredients not listed on the label.
Some products also contained contaminants, including heavy metals and pesticides.
Misleading Claims and Marketing Tactics
Herbal supplement marketing is frequently rife with unproven claims.
Manufacturers often promote their products with anecdotal evidence or pseudo-scientific jargon.
The Federal Trade Commission (FTC) has taken action against several companies for making false or unsubstantiated claims about herbal supplements.
Potential Interactions with Medications
Herbal supplements can interact with prescription medications, leading to adverse effects.
For example, St. John's Wort can interfere with the effectiveness of antidepressants, birth control pills, and other drugs.
Individuals taking medications should always consult with their doctor before using herbal supplements.
Vulnerable Populations at Increased Risk
Certain populations, including pregnant women, children, and the elderly, are particularly vulnerable to the risks of herbal supplements.
The American Academy of Pediatrics advises against the use of herbal supplements in children due to the lack of safety data.
Pregnant women should also avoid herbal supplements unless specifically recommended by their healthcare provider.
Limited Evidence of Efficacy
Despite their popularity, there is limited scientific evidence to support the effectiveness of many herbal supplements.
Many clinical trials are small, poorly designed, or inconclusive.
Consumers should be wary of exaggerated claims and prioritize evidence-based treatments.
The DSHEA of 1994: A Double-Edged Sword
The Dietary Supplement Health and Education Act (DSHEA) of 1994 significantly weakened the FDA's regulatory authority over herbal supplements.
While intended to promote consumer access to dietary supplements, DSHEA has also made it easier for unsafe or ineffective products to enter the market.
Calls for reform of DSHEA are growing among public health advocates.
Reported Adverse Events and Hospitalizations
Adverse events associated with herbal supplements are underreported.
The FDA maintains a database of adverse event reports, but reporting is voluntary, so the true number of incidents is likely much higher.
Some herbal supplements have been linked to serious health problems, including liver damage and kidney failure.
Consumer Protection: What You Can Do
Consumers should be skeptical of claims made about herbal supplements and do their research.
Consult with a healthcare professional before using any herbal supplement, especially if you have underlying health conditions or are taking medications.
Report any adverse events to the FDA's MedWatch program.
The Ongoing Debate: Regulation vs. Access
The debate over the regulation of herbal supplements continues.
Some argue that stricter regulations are needed to protect consumers, while others fear that increased regulation will stifle innovation and limit access to potentially beneficial products.
Ongoing research and public discussion are essential to finding a balance between safety and access.
Next Steps: Increased Vigilance and Research
Increased vigilance from regulatory agencies and greater transparency from manufacturers are crucial.
Further research is needed to evaluate the safety and efficacy of herbal supplements.
Consumers must remain informed and proactive in protecting their health.







:max_bytes(150000):strip_icc()/VWH_Illustration_10-Healing-Herbs-With-Medicinal-Benefits_Illustrator_Mira-Norian_Title_Final-47ce13013375448c9e8e7e8c21fb50f7.jpg)