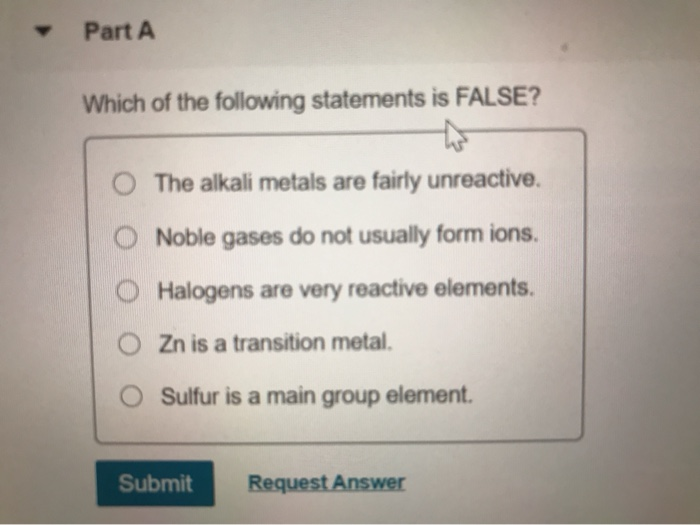
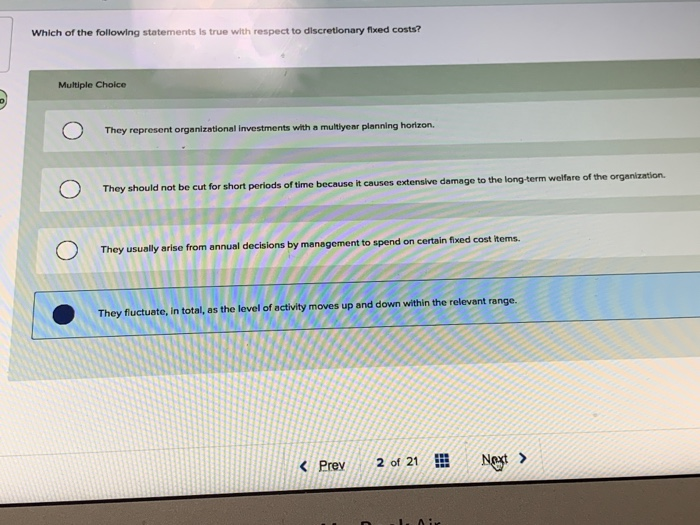
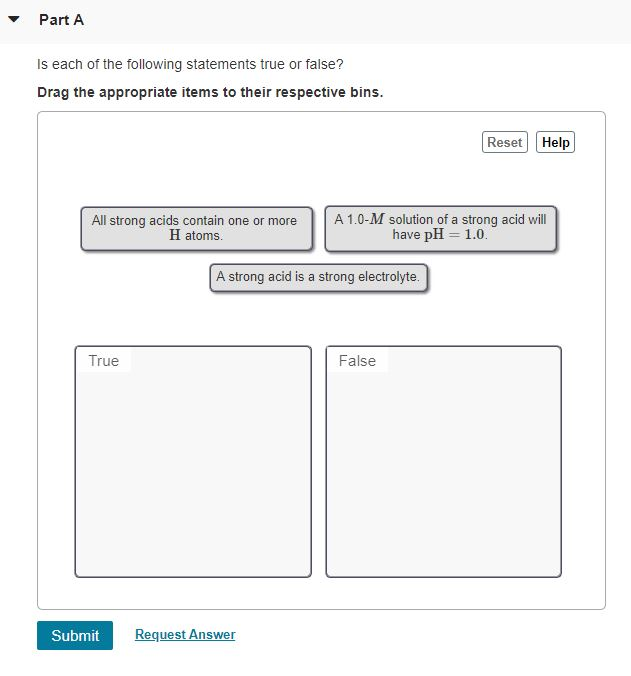
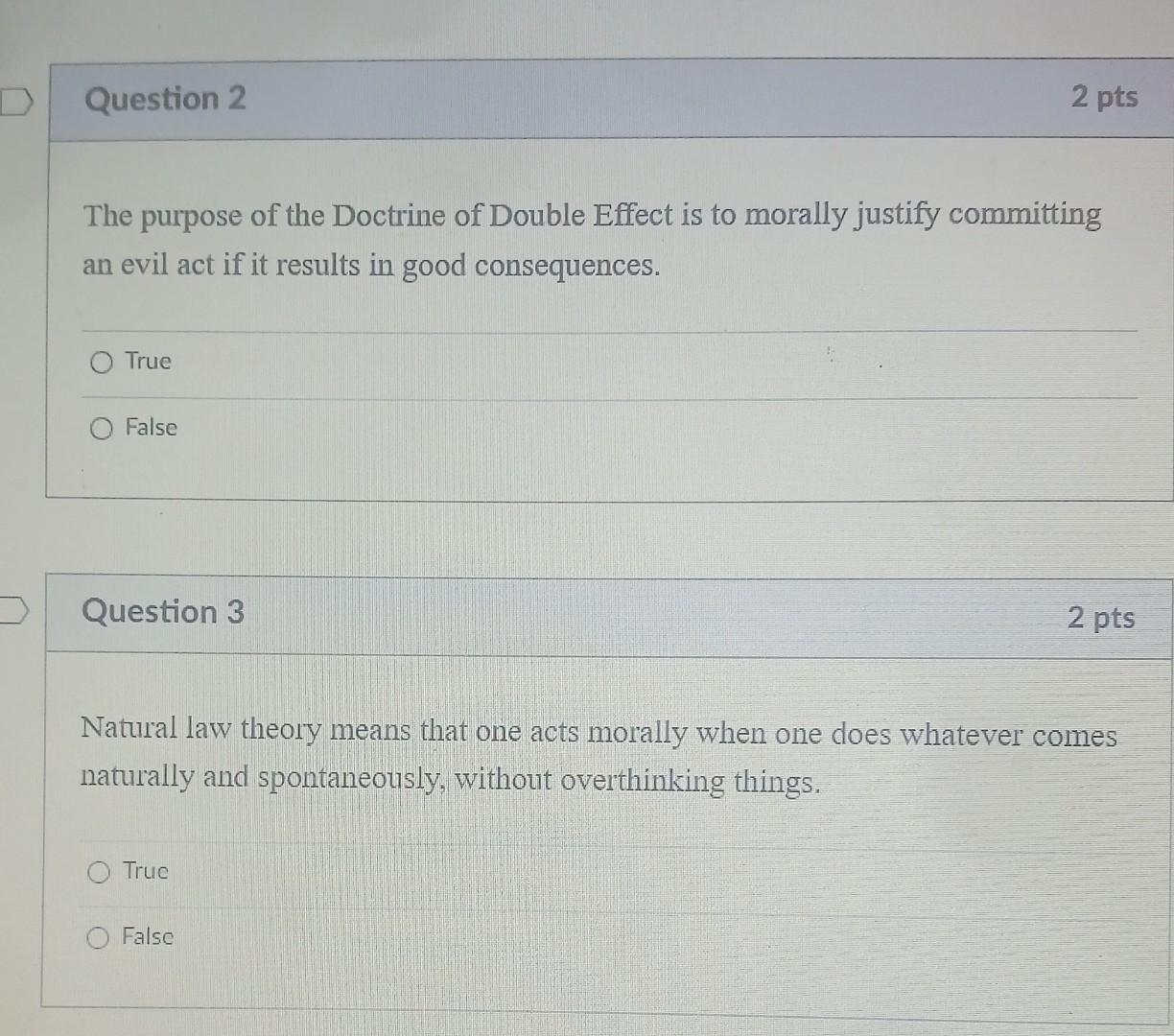
Which Of The Following Statements About Matter Is False

A viral science quiz question is stumping internet users, sparking intense debate and widespread confusion about fundamental concepts of matter. The controversial question, "Which of the following statements about matter is false?", has led to heated discussions and a scramble for accurate information.
The incident highlights a critical gap in public understanding of basic science principles. It also underscores the challenges in disseminating accurate scientific information in a digital age saturated with misinformation.
The Controversial Question
The question presented several statements about matter, one of which was demonstrably false. The ambiguity in wording and the subtle nuances of scientific definitions fueled the confusion. The statements tested knowledge of phase transitions, atomic structure, and the conservation of mass.
Specifically, the options included assertions about:
- The relationship between temperature and the state of matter
- The composition of atoms
- The behavior of matter in different environments
The Correct Answer
While the specific question varies depending on the source, the generally agreed-upon false statement revolved around the indestructibility of matter. The incorrect statement often insinuated that matter can be completely destroyed, violating the principle of conservation of mass.
The principle of conservation of mass, a cornerstone of physics and chemistry, states that matter cannot be created or destroyed. It can only be transformed from one form to another.
This is a critical concept that distinguishes between chemical reactions, where atoms are rearranged, and nuclear reactions, where atoms may undergo changes that change the element.
The Confusion and Debate
Many individuals initially selected other options, demonstrating misunderstandings about phase transitions or the composition of atoms. The nuanced phrasing of the options contributed to the widespread confusion.
Online forums and social media platforms erupted with arguments and justifications for different answers. Experts weighed in, attempting to clarify the correct response and explain the underlying scientific principles.
The debate highlighted the importance of clear and unambiguous language in science education. It also revealed the challenges in conveying complex scientific concepts to a general audience.
Expert Opinions
Dr. Emily Carter, a professor of Chemistry at Princeton University, emphasized the importance of understanding the conservation of mass. "Matter can change forms, but it's not destroyed in typical chemical processes," she clarified in a widely circulated statement.
Other experts pointed to the subtle differences between chemical and nuclear reactions. They stress the impact of context to the statements.
Dr. David Lee, a physics researcher at MIT, explained the intricacies of nuclear processes. He warned against oversimplification. "Nuclear reactions involve different rules than everyday chemistry," he stated.
The Implications
This incident underscores the need for improved science education at all levels. It emphasizes the importance of accurate information dissemination in the digital age.
The widespread confusion highlights the challenges of battling misinformation and promoting scientific literacy. It reinforces the need for critical thinking skills and reliable sources of information.
Addressing scientific misconceptions is crucial for informed decision-making. It also promotes public engagement with science.
Moving Forward
Educational institutions are encouraged to review their curricula to ensure clear explanations of fundamental concepts. Science communicators are urged to use precise language and accessible formats.
Fact-checking organizations are playing a crucial role in debunking misinformation and promoting accurate scientific information. Public awareness campaigns are needed to improve scientific literacy.
The incident serves as a reminder that science is a constantly evolving field. Continuous learning and critical thinking are essential for navigating the complex world around us.


![Which Of The Following Statements About Matter Is False [ANSWERED] Why is the following statement true or false? When a match](https://media.kunduz.com/media/sug-question/raw/59329911-1659632055.9983203.jpeg?h=512)







![Which Of The Following Statements About Matter Is False [ANSWERED] Determine whether the following statement is true or false](https://media.kunduz.com/media/sug-question/raw/56934404-1657559186.410326.jpeg?h=512)
![Which Of The Following Statements About Matter Is False [ANSWERED] Consider the following statements Matter consists of - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210413034320079543-3173394.jpg?h=512)

