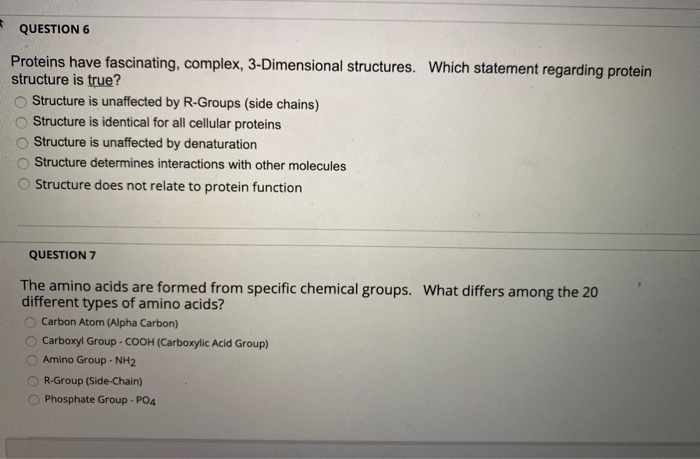
Which Of The Following Statements Concerning Protein Structure Is Incorrect

A widespread misconception regarding protein structure has been identified in recent educational materials, leading to potential errors in understanding fundamental biological processes. The incorrect statement pertains to the hierarchical organization of protein folding, impacting students and researchers alike.
This article dissects the flawed statement, clarifies the accurate protein structure hierarchy (primary, secondary, tertiary, and quaternary), and emphasizes the implications of this misunderstanding across various scientific disciplines. The revelation follows increased scrutiny of online resources and textbooks used in introductory biology and biochemistry courses.
The Erroneous Claim: A Deep Dive
The inaccurate statement commonly found is: "Protein quaternary structure is formed exclusively through disulfide bonds between cysteine residues." This statement misrepresents the true nature of quaternary structure stabilization.
Quaternary structure, the arrangement of multiple polypeptide chains (subunits) in a multi-subunit protein, is stabilized by a variety of interactions. These include, but are not limited to, hydrophobic interactions, hydrogen bonds, ionic interactions (salt bridges), and, in some cases, disulfide bonds.
While disulfide bonds can contribute to quaternary structure, they are not the sole or even primary stabilizing force in many proteins. Many proteins with quaternary structure do not even contain any disulfide bonds.
Understanding the Correct Protein Structure Hierarchy
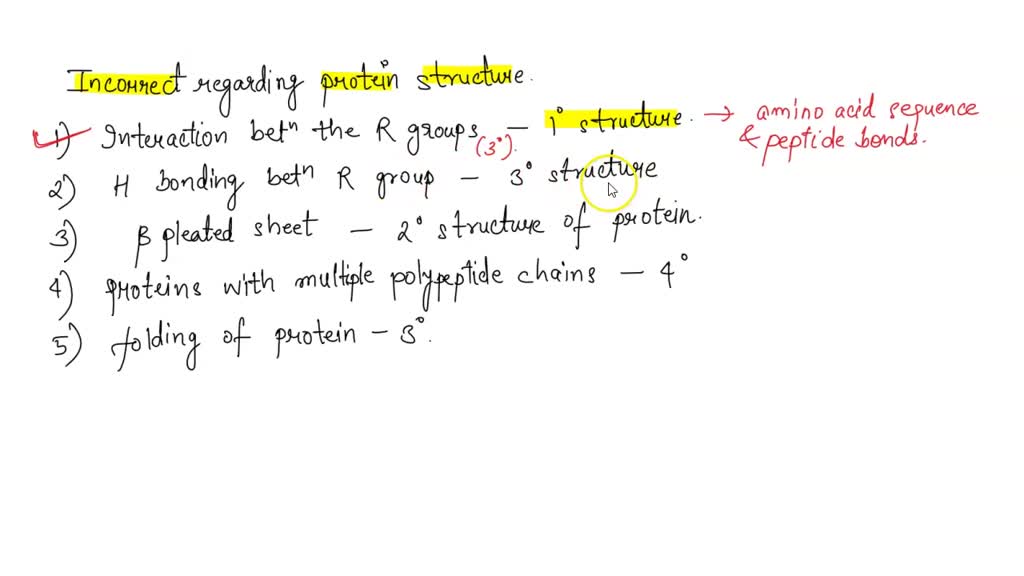
Primary structure: This refers to the linear sequence of amino acids linked by peptide bonds. It's the foundation upon which all other levels of structure are built.
Secondary structure: Localized, repeating patterns of folding such as alpha-helices and beta-sheets are maintained by hydrogen bonds between backbone atoms.
Tertiary structure: The overall three-dimensional shape of a single polypeptide chain, determined by interactions between amino acid side chains (R-groups). These interactions include hydrophobic interactions, hydrogen bonds, disulfide bonds, and ionic bonds.
Quaternary structure: The arrangement of multiple polypeptide chains (subunits) in a multi-subunit protein. As stated before, it is maintained by various non-covalent interactions and, sometimes, covalent disulfide bonds.
The Impact of Misinformation
Believing that disulfide bonds are exclusively responsible for quaternary structure leads to a flawed understanding of protein stability and function. It overlooks the importance of other crucial interactions.
This misconception can hinder understanding of enzyme kinetics, protein-protein interactions, and the effects of mutations on protein structure. Incorrect information negatively impacts students and can affect future scientific research.
Consider hemoglobin, a prime example of a protein with quaternary structure. Hemoglobin is a tetramer, meaning it has four subunits. While some interactions between the subunits might involve weak disulfide bonds under certain rare conditions, the primary interactions holding it together are hydrophobic interactions, hydrogen bonds, and ionic bonds.
Who, What, Where, When, and How
Who: Students, educators, and researchers in biology, biochemistry, and related fields are affected.
What: The incorrect statement regarding protein quaternary structure is "Protein quaternary structure is formed exclusively through disulfide bonds between cysteine residues."
Where: The error is found in various educational materials, including textbooks, online resources, and lectures.
When: The misinformation has been circulating for an undetermined period, but recent scrutiny has brought it to light.
Why: Simplified explanations can sometimes unintentionally lead to inaccuracies. This can be compounded by the spread of misinformation online.
How: By identifying and correcting the error, and by emphasizing the diversity of interactions involved in quaternary structure.
Expert Opinions
"It is crucial to emphasize the multifaceted nature of quaternary structure stabilization," stated Dr. Anna Chen, Professor of Biochemistry at Stanford University. "Disulfide bonds are just one piece of the puzzle."
"The prevalence of this misconception is alarming," added Dr. David Lee, a structural biologist at the National Institutes of Health (NIH). "We need to ensure that students receive accurate information from the outset."
Moving Forward: Corrective Measures
Educational institutions are urged to review their curricula and identify any instances of the incorrect statement. It is important to disseminate correct information regarding protein structure at all levels of education.
Textbook publishers are being contacted to revise their materials. Online resources are being updated to reflect the accurate understanding of protein quaternary structure.
Further research is needed to assess the full extent of the misconception and to develop effective strategies for promoting accurate scientific understanding. This includes developing more interactive and visually engaging materials that illustrate the complex nature of protein folding.










+Which+of+the+following+statements+concerning+unsaturated+fats+is+true..jpg)