Which Of The Following Statements Is Incorrect Regarding Protein Structure

The seemingly simple question, "Which of the following statements is incorrect regarding protein structure?" can reveal surprising gaps in understanding, even among students and professionals in related fields. Recent analysis of quiz and examination data across various educational institutions indicates a persistent struggle with comprehending the nuances of protein folding, levels of organization, and the forces that govern these intricate biological processes.
This article explores the common misconceptions surrounding protein structure, shedding light on the specific areas where knowledge often falters and highlighting the importance of a solid foundation in this fundamental area of biochemistry. Understanding protein structure is not merely an academic exercise; it's crucial for advancements in drug design, understanding disease mechanisms, and developing new biotechnological applications.
The Four Levels of Protein Structure: A Source of Confusion
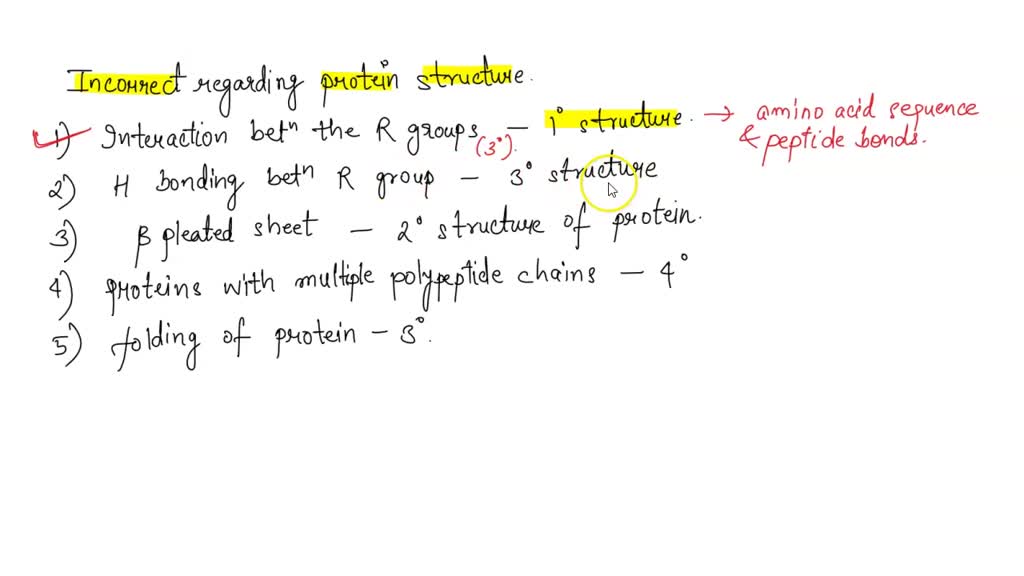
One recurring theme is the confusion surrounding the four levels of protein structure: primary, secondary, tertiary, and quaternary. Many incorrectly identify the forces responsible for maintaining each level, or conflate the definitions of each stage.
Primary Structure: The Amino Acid Sequence
The primary structure refers to the linear sequence of amino acids that make up the polypeptide chain. This sequence, dictated by the genetic code, is held together by covalent peptide bonds.
Errors often arise when students mistakenly attribute hydrogen bonds or hydrophobic interactions to the primary structure, overlooking the critical role of the peptide bond.
Secondary Structure: Local Folding Patterns
Secondary structure describes the local folding patterns within the polypeptide chain, primarily the alpha-helix and beta-sheet. These structures are stabilized by hydrogen bonds between the carbonyl oxygen and amide hydrogen atoms of the peptide backbone.
Common misconceptions include the belief that side chain interactions are the primary driving force in secondary structure formation or a misunderstanding of the specific hydrogen bonding patterns involved.
Tertiary Structure: The Overall 3D Shape
Tertiary structure refers to the overall three-dimensional shape of a single polypeptide chain. This level of structure is determined by a variety of interactions between amino acid side chains, including hydrogen bonds, ionic bonds, hydrophobic interactions, and disulfide bridges.
Often, individuals incorrectly attribute the presence of all these interactions equally in every protein, failing to recognize that the specific amino acid composition and environment will dictate the dominant forces shaping the tertiary structure.
Quaternary Structure: Multi-Subunit Complexes
Quaternary structure applies only to proteins composed of two or more polypeptide chains (subunits). It describes the arrangement and interactions of these subunits to form the functional protein complex. The forces that stabilize quaternary structure are the same as those involved in tertiary structure.
A frequent mistake is the assumption that all proteins possess quaternary structure, neglecting the fact that many proteins function as single polypeptide chains. Understanding the correct definition of quaternary structure and the forces involved is critical.
Forces Governing Protein Folding: Untangling the Web
The question of which forces dictate protein structure invariably leads to a variety of incorrect answers. While all the forces mentioned – hydrogen bonds, hydrophobic interactions, ionic bonds, and disulfide bridges – play a role, their relative importance and the specific contexts in which they operate are often misunderstood.
The hydrophobic effect, in particular, is frequently underestimated or misinterpreted. This effect, driven by the tendency of nonpolar amino acid side chains to cluster together in the interior of the protein, away from water, is a major driving force in protein folding. The drive to maximize entropy among water molecules drives the process.
Misconceptions also surround the formation of disulfide bridges. These covalent bonds, formed between cysteine residues, are important for stabilizing the protein structure, but are not universally present in all proteins.
The Significance of Understanding Protein Structure
Accurate knowledge of protein structure is vital across various scientific disciplines. In drug design, for instance, understanding the three-dimensional structure of a target protein is essential for developing drugs that can bind specifically and effectively.
In the realm of disease research, misfolded proteins are implicated in numerous conditions, including Alzheimer's and Parkinson's diseases. Therefore, a clear understanding of the factors that govern protein folding is crucial for developing therapies to prevent or treat these disorders.
The widespread utilization of protein structure prediction tools further underscores the significance of comprehending this topic. While these tools offer valuable insights, they are only as effective as the user's understanding of the underlying principles and limitations.
Conclusion
The persistent errors in understanding protein structure highlight the need for improved educational strategies. Emphasizing the dynamic nature of protein folding, the relative importance of different forces, and the specific context of each level of organization can significantly enhance comprehension.
By addressing these common misconceptions and fostering a deeper appreciation for the complexities of protein structure, we can better equip future scientists and healthcare professionals to tackle the challenges of drug design, disease research, and biotechnology.
Ultimately, a solid understanding of protein structure is essential for advancing our knowledge of the biological world and developing innovative solutions to improve human health.