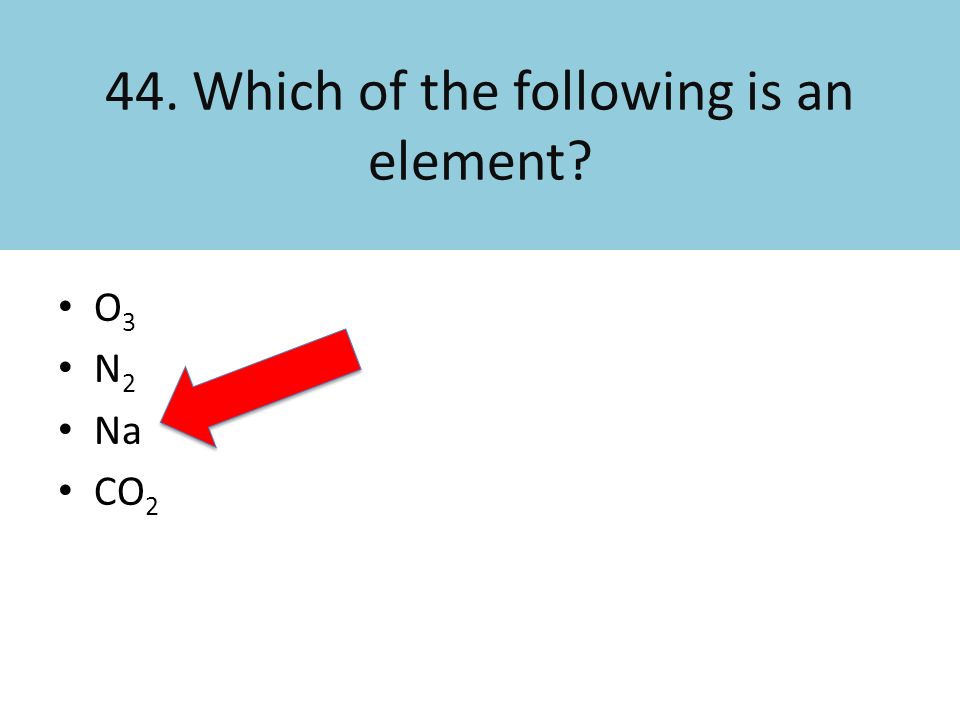
Which Of The Following Would Be Considered An Element

Imagine stepping into a laboratory, the air filled with the subtle scent of chemicals and the quiet hum of scientific instruments. Glass beakers shimmer under the soft glow of overhead lights, each containing a substance with unique properties. Among these are shimmering gold flakes, a block of dull, gray material, and a vial filled with a clear, colorless gas. Which of these, or perhaps something else entirely, constitutes an element in the truest sense of the word?
This seemingly simple question leads us into the fascinating world of chemistry and the fundamental building blocks that make up everything around us. This article will explore what defines an element, differentiate it from other substances, and highlight its crucial role in understanding our world.
Delving into the Definition of an Element
At its core, an element is a pure substance that cannot be broken down into simpler substances by chemical means. This definition is crucial; it sets elements apart from compounds and mixtures, which are formed by the combination of two or more elements.
Consider water, for example. While essential for life, water (H2O) is a compound, formed from the elements hydrogen and oxygen bonded together.
Unlike water, gold (Au) is an element. It can't be chemically broken down into simpler substances while retaining its defining properties.
The Periodic Table: A Map of the Elemental World
The periodic table, a cornerstone of chemistry, organizes all known elements based on their atomic number (the number of protons in their nucleus) and recurring chemical properties. This ingenious arrangement, largely attributed to Dmitri Mendeleev, provides a systematic way to understand and predict the behavior of elements.
Each element occupies a unique spot on the table, symbolized by a one- or two-letter abbreviation (e.g., H for hydrogen, He for helium, O for oxygen). These symbols are internationally recognized, facilitating clear communication within the scientific community.
The periodic table is not merely a list; it's a roadmap revealing relationships between elements, grouping them by shared characteristics and electronic structures. This structure enables scientists to predict how elements will interact and form compounds.
Distinguishing Elements from Compounds and Mixtures
Understanding the difference between elements, compounds, and mixtures is essential for grasping basic chemistry. As mentioned earlier, compounds are substances formed when two or more elements chemically combine in a fixed ratio.
For example, table salt (sodium chloride, NaCl) is a compound formed from the elements sodium (Na) and chlorine (Cl). Its properties are distinct from those of its constituent elements.
Mixtures, on the other hand, are combinations of substances that are physically combined but not chemically bonded. Air, for instance, is a mixture of nitrogen, oxygen, argon, and other gases.
Unlike compounds, mixtures can be separated by physical means, such as filtration or evaporation, without altering the chemical nature of the individual components. A salad, for example, is a mixute.
Examples of Elements and Their Significance
The elements are incredibly diverse, each with unique properties and applications. Oxygen (O), essential for respiration, supports life as we know it.
Carbon (C), with its remarkable ability to form diverse bonds, is the backbone of organic chemistry, the study of life itself.
Iron (Fe), a strong and versatile metal, is crucial for construction and is found in hemoglobin, the oxygen-carrying molecule in our blood.
Silicon (Si) is indispensable in the manufacture of semiconductors. Semiconductors are the foundation of our modern digital world.
Identifying an Element: Key Properties to Consider
Determining whether a substance is an element often involves examining its properties. Elements possess unique characteristics that can be used for identification. These include melting point, boiling point, density, and chemical reactivity.
For example, gold is known for its characteristic yellow color, high density, and resistance to corrosion. These properties distinguish it from other metals.
Spectroscopy, a powerful analytical technique, can also identify elements by analyzing the light they emit or absorb. Each element has a unique spectral fingerprint, acting as a reliable marker.
The Ongoing Discovery of New Elements
While the first elements were discovered centuries ago, the quest for new elements continues. Scientists synthesize elements in particle accelerators, pushing the boundaries of nuclear physics.
These new elements, often radioactive and extremely short-lived, expand our understanding of nuclear structure and the limits of the periodic table.
The most recent additions to the periodic table have been elements with atomic numbers 113 (nihonium), 115 (moscovium), 117 (tennessine), and 118 (oganesson), all artificially created.
The Importance of Elements in Our World
Elements are not just abstract concepts confined to laboratories; they are integral to every aspect of our lives. They form the foundation of everything from the air we breathe to the materials we use to build our homes.
Understanding the properties of elements is crucial for advancements in medicine, engineering, and environmental science. New technologies depend on our ability to manipulate and combine elements in innovative ways.
The study of elements fosters a deeper appreciation for the interconnectedness of the natural world and our place within it.
In Conclusion: The Enduring Significance of Elements
So, returning to our initial scenario, the gold flakes, if pure, represent a clear example of an element. The gray block could potentially be an element as well, depending on its composition and purity, while the clear gas would need further analysis to determine its elemental nature.
The elements are the foundational building blocks of our universe, a testament to the elegance and order inherent in the natural world. By grasping the concept of an element, we unlock a deeper understanding of the composition, properties, and transformations of matter.
As we continue to explore the elemental world, we not only expand our scientific knowledge but also deepen our connection to the fundamental constituents of our existence.
+He%2C+F2%2C+HCl%2C+S8..jpg)










![Which Of The Following Would Be Considered An Element [ANSWERED] Consider the following table Element Atomic number A B C D](https://media.kunduz.com/media/sug-question-candidate/20210909164556981345-3522319.jpg?h=512)

![Which Of The Following Would Be Considered An Element [ANSWERED] What is the symbol for the following element Atomic Number](https://media.kunduz.com/media/sug-question-candidate/20220619203541153394-3498554.jpg?h=512)

