Which Process Reduces Molecular Oxygen To Water

Imagine a crisp autumn morning. Dew clings to the blades of grass, and the air is filled with the scent of damp earth and decaying leaves. A robin, perched on a branch, puffs out its chest and sings a cheerful melody. Unseen, within every cell of that robin, and within every cell of every living thing that breathes air, a remarkable process is occurring, a microscopic marvel that sustains life itself.
At the heart of this marvel is a process called the electron transport chain, specifically the final step catalyzed by the enzyme cytochrome c oxidase. This process reduces molecular oxygen (O2) to water (H2O), a seemingly simple reaction with profound implications for energy production and the continuation of life as we know it.
The Electron Transport Chain: A Cellular Powerhouse
To understand the reduction of oxygen to water, we must first delve into the world of cellular respiration. This is the process by which organisms convert nutrients into usable energy, primarily in the form of ATP (adenosine triphosphate), the cell's energy currency.
Cellular respiration comprises several stages, including glycolysis, the citric acid cycle (also known as the Krebs cycle), and the electron transport chain. The electron transport chain is located in the inner mitochondrial membrane, the powerhouse of the cell.
This chain is a series of protein complexes that pass electrons from one molecule to another. These electrons originate from the breakdown products of glucose and other fuels.
The Role of Cytochrome c Oxidase
Cytochrome c oxidase (Complex IV) stands as the final protein complex in the electron transport chain. It's a large, intricate enzyme containing metal ions, including copper and iron, which are crucial for its function.
This enzyme accepts electrons from cytochrome c, another mobile electron carrier in the chain. These electrons, once accepted, are used to drive the reduction of molecular oxygen.
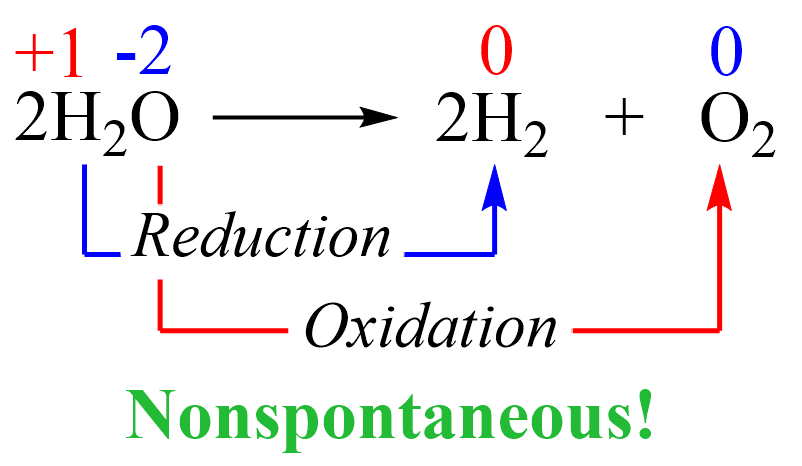
The overall reaction catalyzed by cytochrome c oxidase can be summarized as: O2 + 4H+ + 4e- → 2H2O. This reaction uses four protons (H+) and four electrons (e-) to convert one molecule of oxygen into two molecules of water.
This process is not just about creating water; it's also intricately linked to the generation of a proton gradient across the inner mitochondrial membrane. This proton gradient, in turn, powers the synthesis of ATP through a process called oxidative phosphorylation.
Why is This Reduction So Important?
The reduction of oxygen to water by cytochrome c oxidase is arguably the most crucial step in aerobic respiration. Without it, the electron transport chain would grind to a halt, and ATP production would dramatically decrease.
This dramatic decrease in ATP production would be catastrophic for most organisms. Cells require a constant supply of ATP to perform essential functions, such as muscle contraction, nerve impulse transmission, and protein synthesis.
In fact, cyanide, a notorious poison, exerts its toxic effects by binding to cytochrome c oxidase and inhibiting its function. This effectively shuts down cellular respiration, leading to rapid cell death.
The efficiency of this process is also remarkable. Cytochrome c oxidase performs the four-electron reduction of oxygen with high fidelity. This minimizes the formation of harmful reactive oxygen species (ROS), such as superoxide and hydrogen peroxide.
These ROS can damage cellular components, including DNA, proteins, and lipids. Uncontrolled ROS production is implicated in aging and various diseases.
Evolutionary Significance
The evolution of cytochrome c oxidase was a pivotal event in the history of life on Earth. It allowed organisms to harness the full potential of oxygen as a terminal electron acceptor in respiration.
Oxygen is a powerful oxidizing agent, and its use in respiration yields significantly more energy than anaerobic processes. This evolutionary adaptation enabled the development of complex, multicellular life forms.
Studies suggest that cytochrome c oxidase arose relatively early in the evolution of prokaryotes. From there it was eventually incorporated into eukaryotic cells through the endosymbiotic event that also gave rise to mitochondria.
Beyond Energy Production: Other Roles of Oxygen Reduction
While the primary function of oxygen reduction by cytochrome c oxidase is energy production, this process also plays a role in other cellular functions. For example, it participates in regulating cellular signaling pathways.
The enzyme's activity can be influenced by various factors, including oxygen levels, the availability of reducing equivalents, and the presence of regulatory molecules. These regulatory mechanisms allow cells to fine-tune their energy production in response to changing environmental conditions.
Research also indicates that cytochrome c oxidase may be involved in thermogenesis, the process of heat production. In certain tissues, such as brown adipose tissue, the enzyme can uncouple proton pumping from ATP synthesis, dissipating energy as heat.
Future Directions and Research
Scientists continue to investigate the intricate details of cytochrome c oxidase and its role in cellular respiration. Researchers are studying the enzyme's structure, function, and regulation, as well as its involvement in disease.
Understanding the molecular mechanisms of cytochrome c oxidase is crucial for developing new therapies for mitochondrial disorders, cancer, and other diseases. For instance, targeting cytochrome c oxidase could be a potential strategy for selectively inhibiting cancer cell growth.
Moreover, researchers are exploring the possibility of using cytochrome c oxidase as a biosensor. Its ability to detect oxygen levels could be harnessed to develop devices for monitoring oxygen saturation in biological samples.
The study of cytochrome c oxidase also offers insights into the evolution of life and the intricate workings of biological systems. By unraveling the mysteries of this remarkable enzyme, we gain a deeper appreciation for the complexity and elegance of the natural world.
In the end, the seemingly simple act of reducing molecular oxygen to water is a testament to the power of evolution and the ingenuity of nature. It's a process that underpins the very existence of aerobic life, a silent, microscopic symphony playing out within every breath we take.