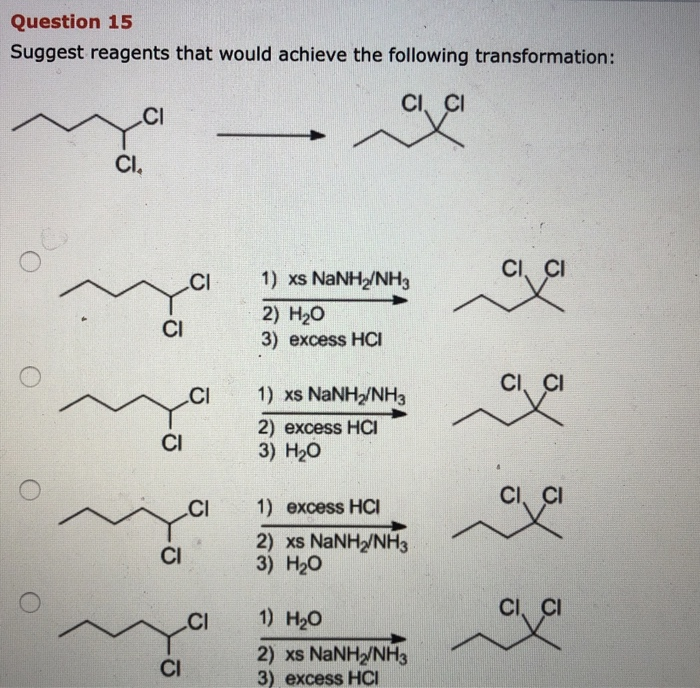
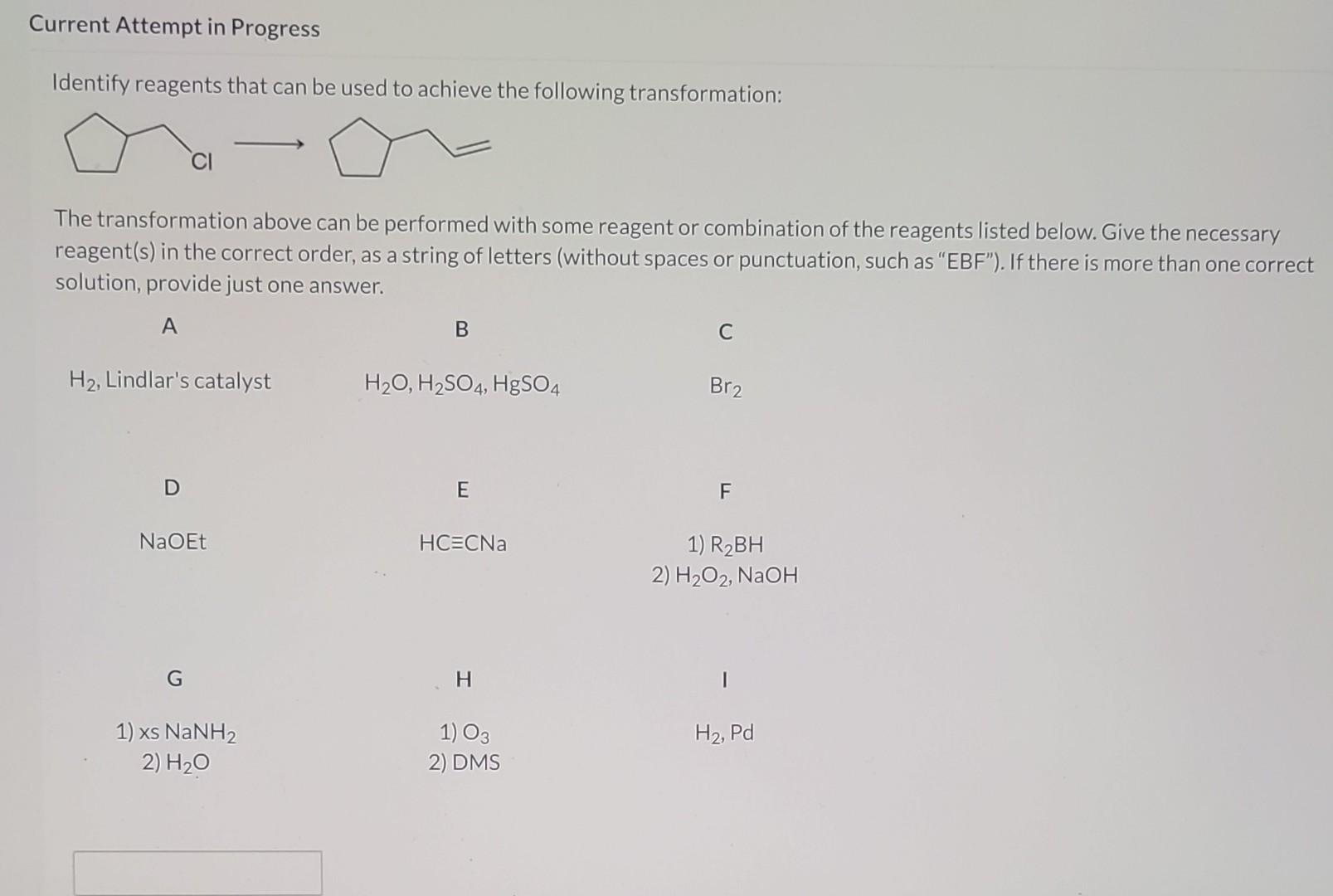
Which Reagents Will Achieve The Following Transformation
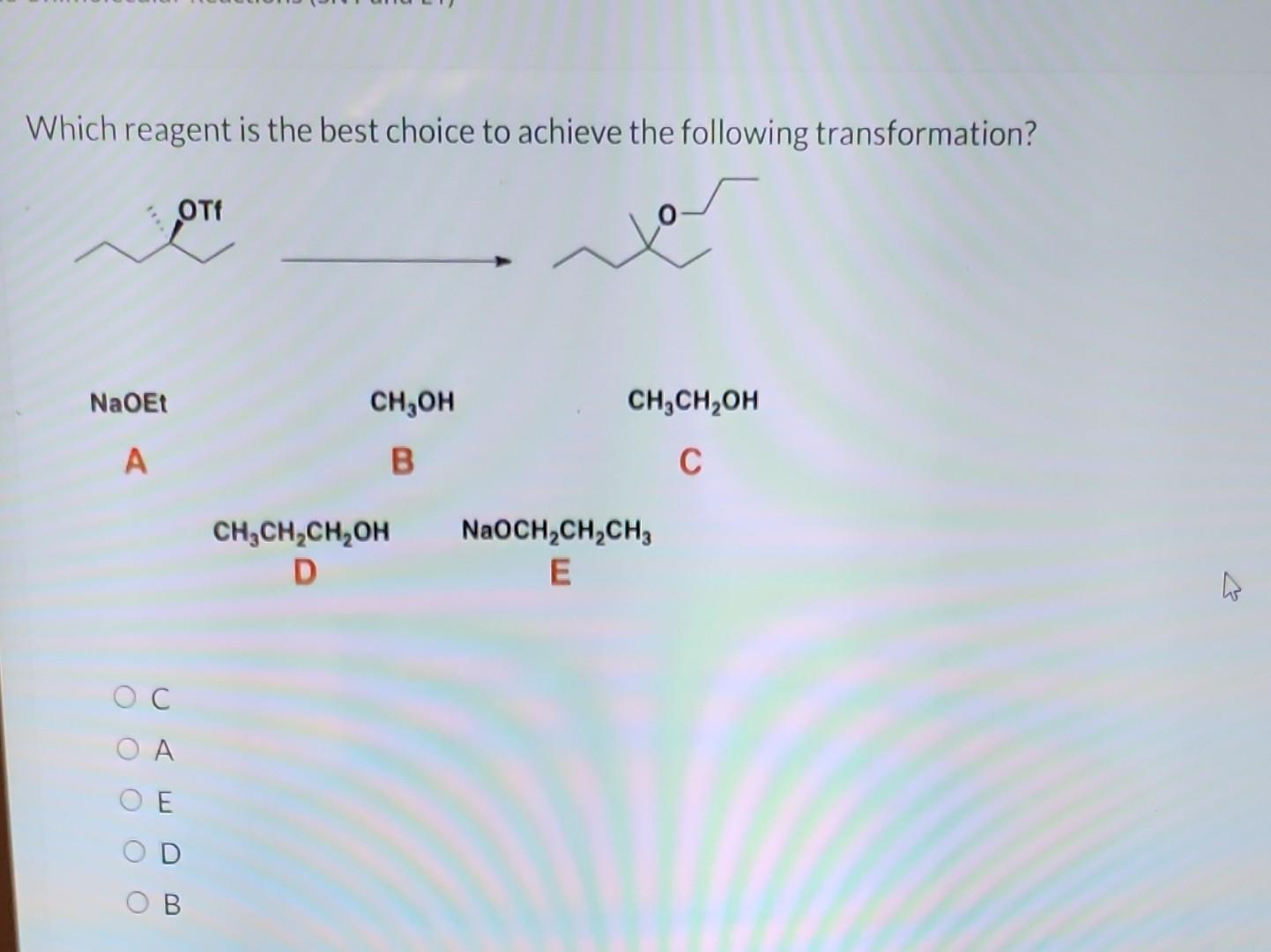
Organic chemists frequently face the challenge of selectively transforming functional groups on complex molecules. A common query arises: "Which reagents will achieve the following transformation?" This question often necessitates careful consideration of reaction mechanisms, protecting groups, and potential side reactions.
Understanding the appropriate reagents to accomplish a specific chemical transformation is crucial for success in organic synthesis. This article explores the factors influencing reagent selection and highlights common strategies employed by chemists.
The Significance of Reagent Selection
The choice of reagents is paramount in organic synthesis. The wrong reagent can lead to undesired products, low yields, or even complete failure of the reaction.
Selecting the right reagents can be a complex process, influenced by several factors. This includes the nature of the starting material, the desired product, and the presence of other functional groups in the molecule.
Key Considerations
Before choosing a reagent, chemists must carefully analyze the reaction. This includes identifying the functional group being transformed and any potential side reactions that could occur.
Protecting groups are often employed to prevent unwanted reactions at other sites in the molecule. These groups temporarily mask a functional group, allowing the desired transformation to proceed selectively.
The reaction mechanism is another crucial consideration. Understanding how the reaction proceeds allows chemists to predict the outcome and select reagents that will favor the desired pathway.
Common Reagents and Strategies
Numerous reagents are available to achieve specific chemical transformations. Some of the most common reactions include oxidation, reduction, and carbon-carbon bond formation.
Oxidizing agents, such as potassium permanganate (KMnO4) and chromium trioxide (CrO3), are used to increase the oxidation state of a molecule. These reagents can convert alcohols to aldehydes or ketones, and further oxidize aldehydes to carboxylic acids.
Reducing agents, such as sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4), are used to decrease the oxidation state of a molecule. NaBH4 selectively reduces ketones and aldehydes to alcohols, while LiAlH4 is a stronger reducing agent that can reduce carboxylic acids, esters, and amides to alcohols and amines.
Grignard reagents (RMgX) and organolithium reagents (RLi) are powerful nucleophiles that can form carbon-carbon bonds. These reagents react with carbonyl compounds to form alcohols, and can also be used in a variety of other coupling reactions.
Wittig reaction utilizes a phosphorus ylide to convert a ketone or aldehyde to an alkene. This is a powerful method for forming carbon-carbon double bonds with high stereoselectivity.
Catalytic hydrogenation uses hydrogen gas (H2) in the presence of a metal catalyst (e.g., Pd, Pt, Ni) to reduce alkenes and alkynes to alkanes. It is a widely used method for saturating unsaturated hydrocarbons.
Case Studies: Examples in Action
Consider the transformation of a primary alcohol to a carboxylic acid. This transformation typically requires a strong oxidizing agent such as potassium permanganate or chromic acid.
If the molecule contains an alkene, it might be necessary to protect the alkene functionality. This can be achieved using a protecting group, such as a silyl ether, which is resistant to oxidation.
Another example is the conversion of a ketone to a secondary alcohol. This transformation can be accomplished using a reducing agent like sodium borohydride.
If the ketone is part of a more complex molecule with other sensitive functional groups, milder reducing agents or protecting groups might be needed. This is where knowledge of reagent selectivity becomes especially important.
The creation of a new carbon-carbon bond is exemplified by the formation of a Grignard reagent. This involves reacting an alkyl or aryl halide with magnesium metal in anhydrous ether.
The resulting Grignard reagent can then be reacted with a variety of electrophiles. Electrophiles include carbonyl compounds to create new carbon-carbon bonds and generate alcohols.
The Impact on Society and Research
The ability to selectively transform functional groups is crucial for developing new drugs and materials. Many pharmaceutical compounds require complex synthetic routes to achieve the desired structure.
Efficient reagent selection is also important for reducing waste and improving the sustainability of chemical processes. Green chemistry principles emphasize the use of environmentally friendly reagents and minimizing the generation of byproducts.
Advances in reagent development are constantly pushing the boundaries of what is possible in organic synthesis. New catalysts and reagents are enabling chemists to perform more complex and selective transformations.
Conclusion
The question of which reagents will achieve a particular transformation is a fundamental one in organic chemistry. By carefully considering the reaction mechanism, protecting groups, and potential side reactions, chemists can select the most appropriate reagents for the job.
Understanding the principles of reagent selection is crucial for success in both research and industrial applications. It is the key to unlocking new possibilities in chemistry and improving the quality of life.
Continued innovation in reagent development will continue to drive progress in various fields. These fields include medicine, materials science, and energy.