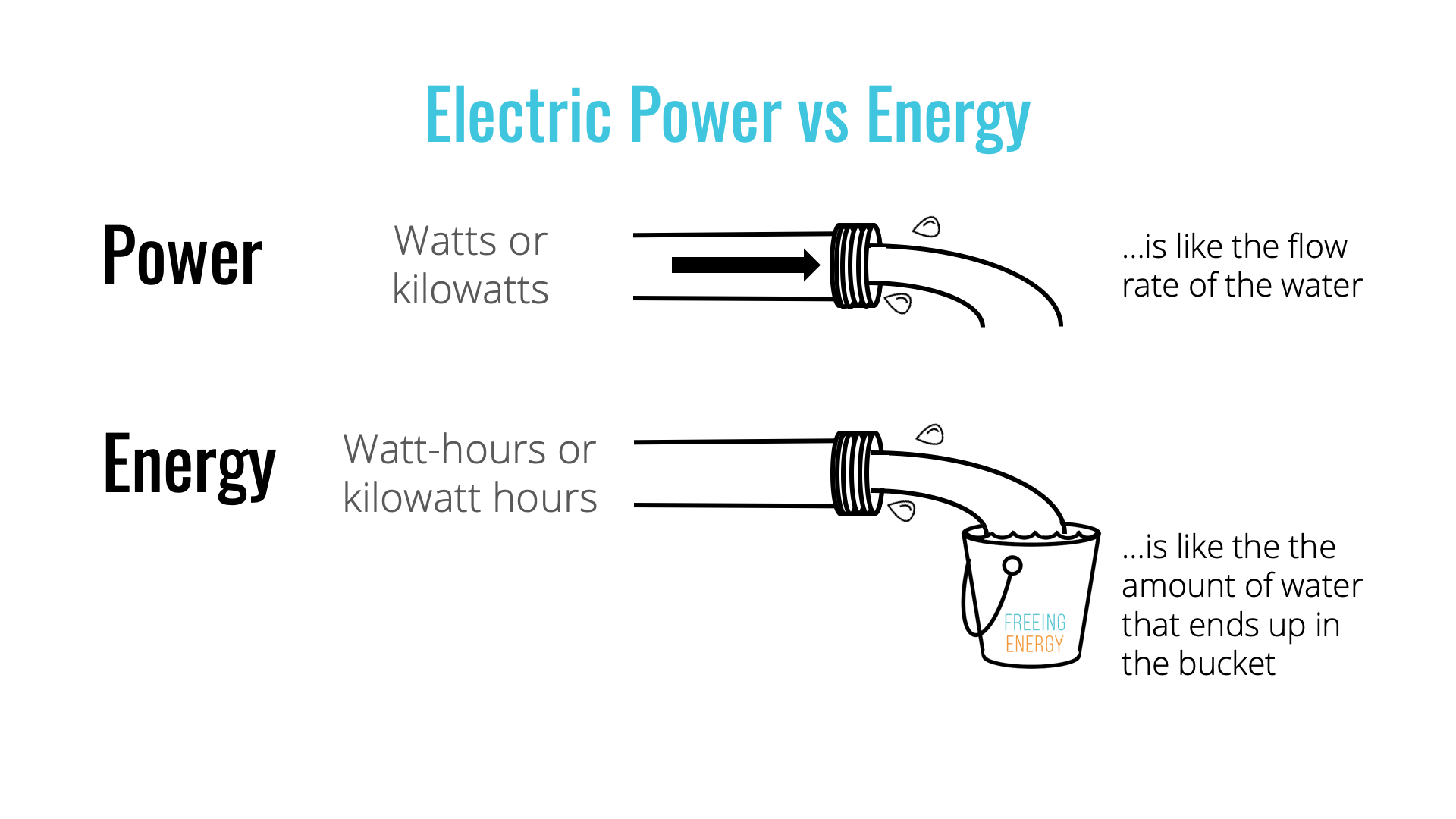
Which Term Means The Amount Of Energy In Water
The invisible force governing the lifeblood of our planet, water, holds a secret quantifiable by a single, critical term. That term dictates not only the potential for immense power but also the delicate balance of ecosystems and the very stability of our climate. Identifying this key term is crucial for understanding everything from hydroelectric energy to the melting of polar ice caps.
The term that describes the amount of energy in water is thermal energy, often described in terms of temperature and measured as heat. While seemingly straightforward, the concept and its various manifestations (such as latent heat and specific heat capacity) are integral to numerous scientific disciplines and practical applications. Understanding thermal energy in water is essential for managing water resources, predicting weather patterns, and developing sustainable energy solutions.
Understanding Thermal Energy
Thermal energy is the internal energy of a system due to the kinetic energy of its atoms and molecules. In the case of water, this energy is manifested in the constant motion of water molecules. The faster these molecules move, the higher the temperature and thus the greater the thermal energy.
It’s crucial to distinguish between temperature and heat. Temperature is a measure of the average kinetic energy of the molecules, while heat is the transfer of thermal energy between objects or systems at different temperatures. Thus, a large lake at a moderate temperature can contain significantly more thermal energy than a small cup of boiling water.
Specific Heat Capacity
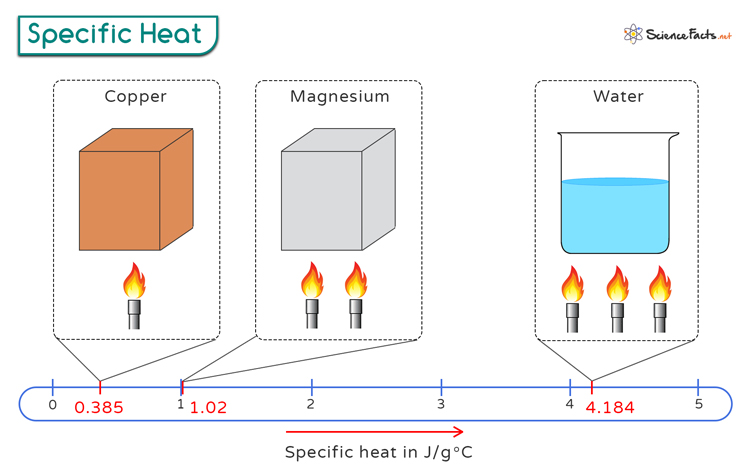
Water's unique properties stem largely from its high specific heat capacity. This means water can absorb a large amount of heat without a significant change in temperature. This characteristic is vital for regulating Earth's climate and allows aquatic ecosystems to remain relatively stable despite fluctuations in air temperature.
According to the U.S. Geological Survey (USGS), water’s high specific heat helps moderate coastal climates. It also prevents drastic temperature swings in lakes and oceans. This ensures a stable environment for marine life.
Latent Heat
Water also possesses a high latent heat of fusion and vaporization. This refers to the energy required to change its state – from solid to liquid (melting) or from liquid to gas (vaporization) – without changing temperature. When ice melts, it absorbs a significant amount of thermal energy, cooling its surroundings.
Similarly, when water evaporates, it absorbs heat from the environment, providing a natural cooling effect. This principle is utilized in many cooling technologies, from evaporative coolers to industrial cooling processes.
Implications for Climate and Environment
The thermal energy content of water plays a central role in global climate regulation. Oceans act as massive heat sinks, absorbing and redistributing thermal energy around the planet. This process influences weather patterns and ocean currents.
Increased atmospheric greenhouse gases are causing the oceans to absorb more thermal energy. This leads to rising sea temperatures, which has cascading effects on marine ecosystems. Warmer waters can lead to coral bleaching, changes in fish migration patterns, and increased frequency and intensity of marine heatwaves.
The National Oceanic and Atmospheric Administration (NOAA) has documented a significant increase in ocean heat content over the past several decades. They link this directly to human-caused climate change. This excess heat is contributing to sea-level rise through thermal expansion and melting glaciers and ice sheets.
Thermal Energy in Water and Energy Production
Water's thermal energy can be harnessed to generate electricity. Hydrothermal energy utilizes the temperature difference between deep ocean water and surface water. This process can drive turbines to produce electricity.
While not as widely used as other renewable energy sources, hydrothermal energy has the potential to provide a sustainable and clean energy source. It's especially appealing in regions with access to deep ocean resources.
Hydropower, another well-established method, utilizes the kinetic energy of moving water, but temperature still plays a role. Changes in water temperature can affect the efficiency of hydropower plants and the surrounding ecosystems.
Challenges and Future Research
Measuring and predicting changes in the thermal energy content of water present ongoing challenges. Scientists use a combination of satellite data, ocean buoys, and climate models to track ocean temperatures and heat distribution.
Improving the accuracy of these models is crucial for predicting future climate scenarios and developing effective mitigation strategies. Continued research is also needed to understand the complex interactions between thermal energy, ocean currents, and atmospheric processes.
Further exploration into advanced hydrothermal technologies and sustainable water management practices is essential. This can mitigate the impacts of rising water temperatures and ensure the responsible use of this vital resource.
In conclusion, the term thermal energy encapsulates the amount of energy residing within water, influencing everything from global climate patterns to energy production. A deep understanding of its dynamics is paramount. This understanding is critical for addressing the challenges of a changing climate and ensuring a sustainable future.