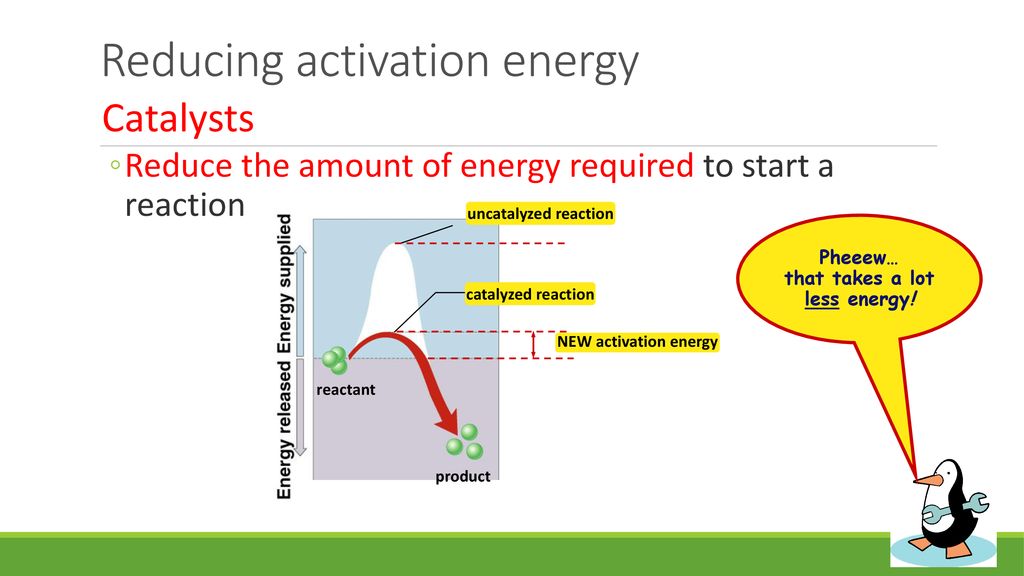
Why Is Reaching Activation Energy Necessary
The seemingly simple act of striking a match, starting a car engine, or even the biochemical reactions within our own bodies all hinge on a fundamental principle: activation energy. This invisible barrier determines whether a reaction, thermodynamically favorable as it may be, actually occurs.
Without sufficient activation energy, the world as we know it would grind to a halt. But why is this energetic hurdle so critical, and what are the implications of its existence?
The Necessity of the Activation Energy Barrier
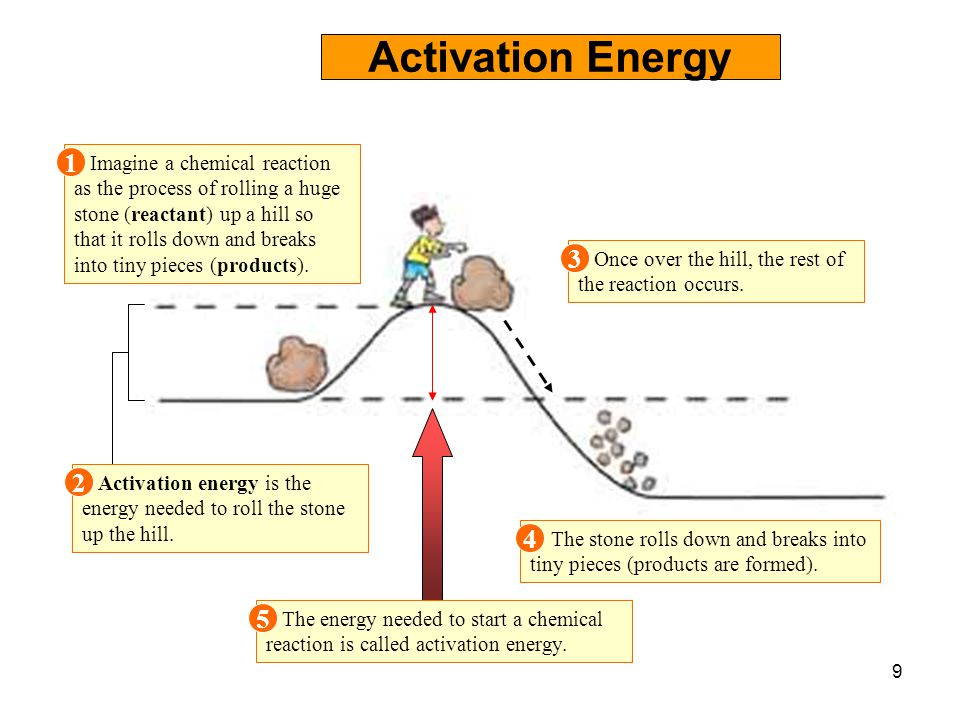
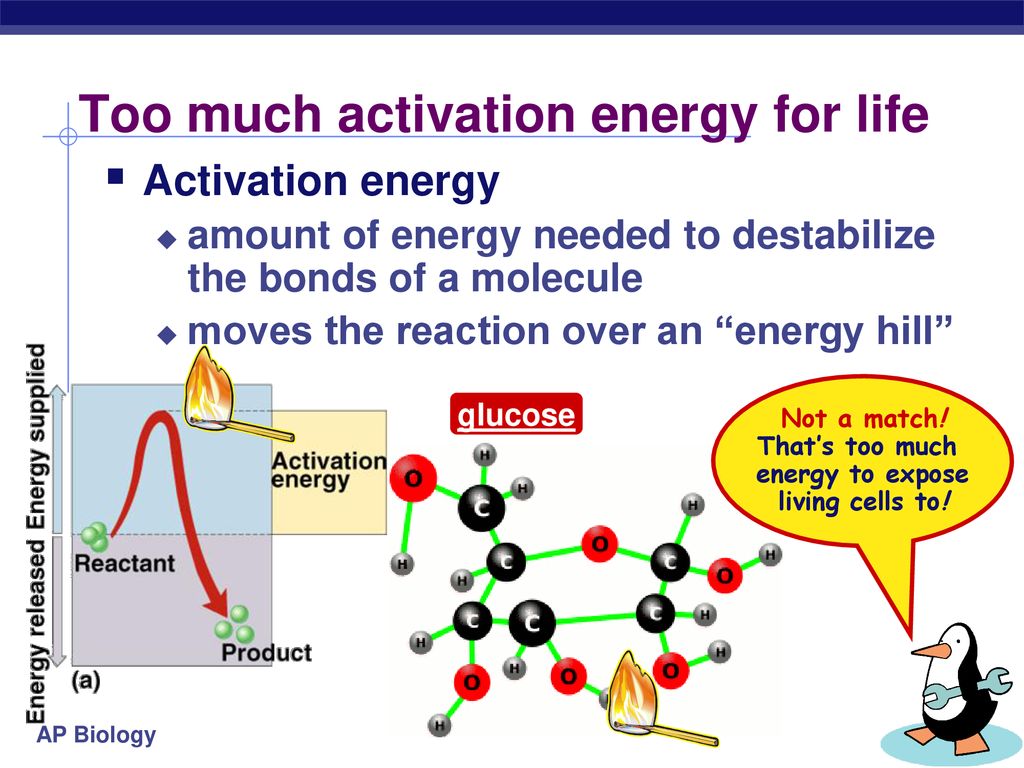
At its core, activation energy is the minimum energy required for a chemical reaction to begin. This "energy barrier" is not arbitrary; it represents the energy needed to break existing chemical bonds, stretch them to a transition state, and initiate the formation of new bonds.
Without this barrier, reactions would occur spontaneously and instantaneously. Such a scenario would be chaotic and uncontrollable, making complex life and stable chemical systems impossible.
Stability and Control
The existence of activation energy provides stability to the chemical world. Imagine a can of gasoline; it's a highly energetic substance capable of rapid oxidation (burning).
However, it remains relatively inert until a spark provides the necessary activation energy, initiating the combustion reaction. This inherent stability is vital for storing energy and controlling chemical processes.
"Activation energy ensures that not every reaction happens all the time. It's the gatekeeper that allows us to selectively initiate and control chemical transformations," explains Dr. Emily Carter, a professor of Chemical and Biomolecular Engineering at Princeton University.
Enzymes and Biological Processes
Within living organisms, activation energy plays an even more crucial role. Biochemical reactions, vital for life, often have high activation energies.
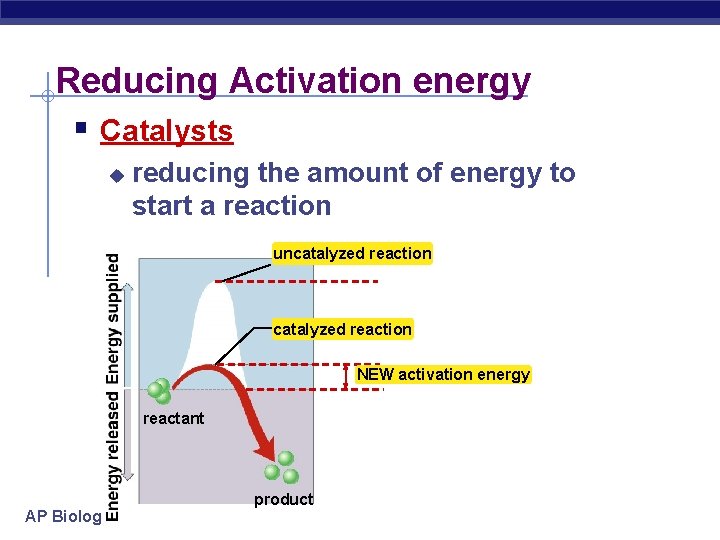
To overcome these barriers, organisms rely on enzymes, biological catalysts that lower the activation energy of specific reactions, allowing them to occur at biologically relevant temperatures.
Enzymes don't change the overall thermodynamics of a reaction, but they drastically increase the reaction rate, making life as we know it possible. Without enzymes, metabolic processes would be too slow to sustain life.
Industrial Applications
Understanding and manipulating activation energy is paramount in various industrial processes. Chemical engineers strive to optimize reaction conditions to minimize the required activation energy, thereby increasing reaction yields and reducing energy consumption.
Catalysts, similar to enzymes, are widely used in industrial settings to lower activation energies and accelerate chemical reactions. For example, the Haber-Bosch process, which produces ammonia for fertilizers, relies on an iron catalyst to lower the activation energy of nitrogen fixation.
The process normally requires harsh conditions and high temperatures, but with the introduction of catalyst, the reaction is much more efficient.
The Transition State: A Key Concept
Activation energy is intrinsically linked to the concept of the transition state. This is the highest energy point along the reaction pathway, representing an unstable intermediate configuration where bonds are partially broken and partially formed.
The difference in energy between the reactants and the transition state is the activation energy. Visualizing and understanding the transition state is crucial for developing catalysts and controlling reaction rates.
Computational chemistry and advanced spectroscopic techniques are increasingly used to probe the transition state and design more efficient catalysts.
Arrhenius Equation: Quantifying the Relationship
The relationship between activation energy and reaction rate is quantified by the Arrhenius equation. This equation states that the rate constant of a reaction is exponentially dependent on the activation energy and temperature.
A higher activation energy results in a slower reaction rate, while an increase in temperature increases the reaction rate by providing more molecules with sufficient energy to overcome the activation barrier.
The Arrhenius equation is a cornerstone of chemical kinetics and provides a framework for predicting and controlling reaction rates.
Challenges and Future Directions
While the concept of activation energy is well-established, challenges remain in accurately predicting and controlling it, particularly in complex systems.
Developing catalysts that can selectively lower activation energies for specific reactions is an ongoing area of research. This would have significant implications for various fields, including sustainable energy, pharmaceuticals, and materials science.
Furthermore, understanding the role of quantum mechanical effects in determining activation energies is becoming increasingly important, especially for reactions involving light atoms like hydrogen.
Quantum tunneling, where particles can pass through energy barriers, can significantly alter reaction rates, particularly at low temperatures.
Conclusion
Activation energy, though often unseen, is an indispensable component of the chemical and biological world. It provides stability, enables control, and underlies the intricate dance of chemical transformations that sustain life and drive industrial processes.
By continuing to deepen our understanding of activation energy and develop innovative ways to manipulate it, we can unlock new possibilities in various fields and address some of the world's most pressing challenges.
From designing more efficient catalysts to developing new energy technologies, the future of chemistry and related fields hinges on our ability to master this fundamental energetic barrier.
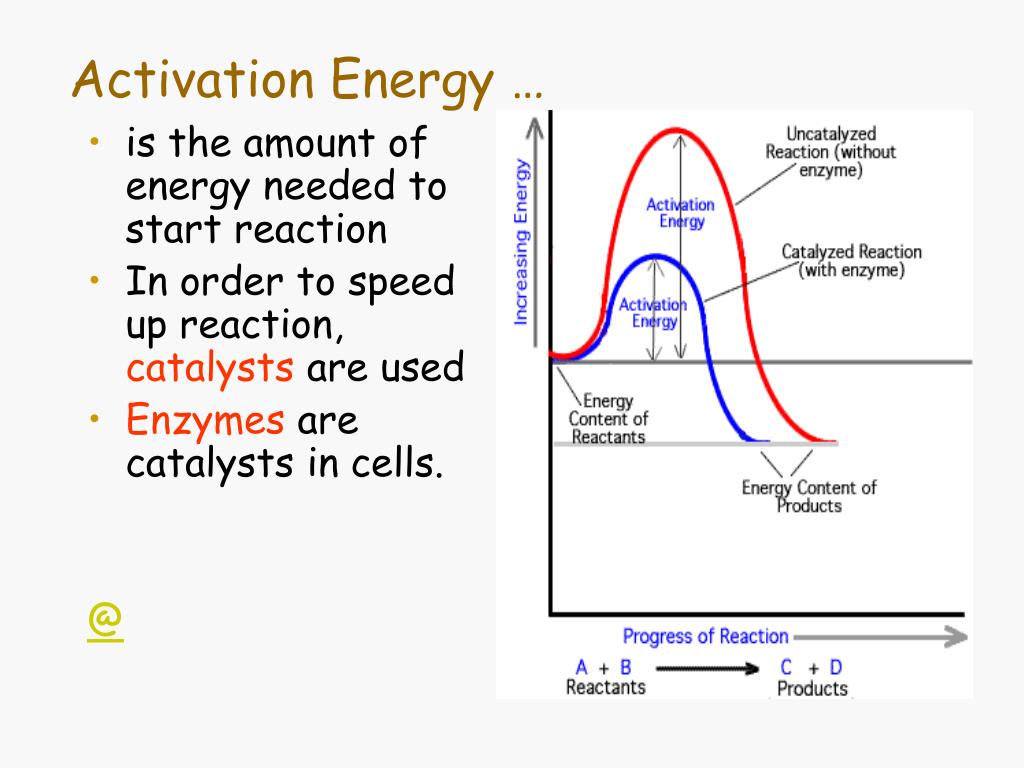



.jpg)


–+minimum+energy+required+to+transform+reactants+into+the+activated+complex..jpg)
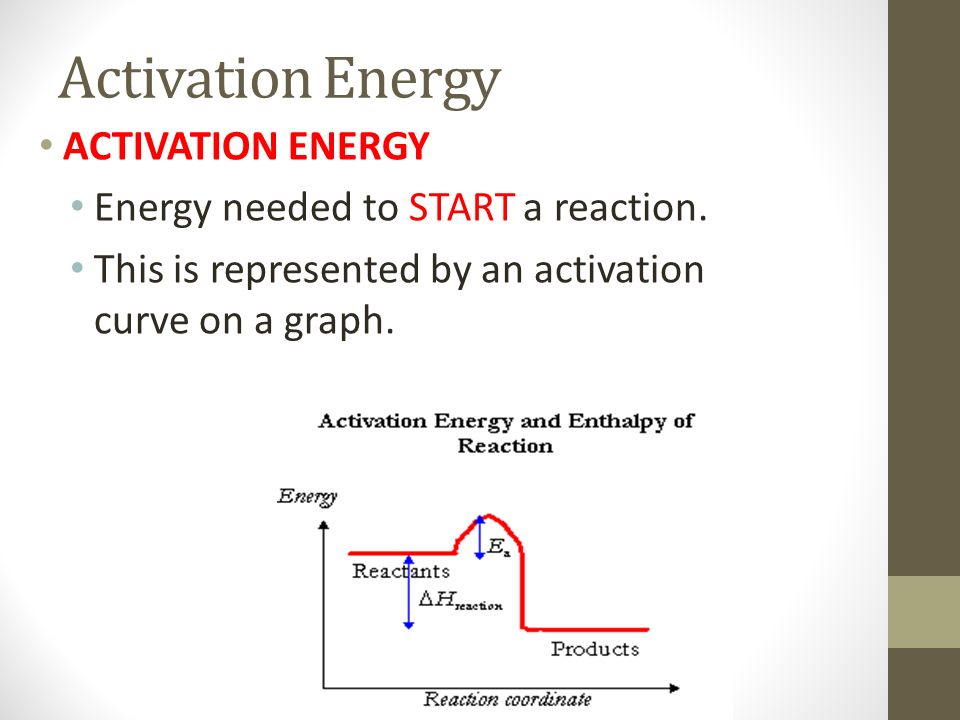



.jpg)