Writing And Balancing Complex Half-reactions In Acidic Solution

Breakthrough simplifies the balancing of complex oxidation-reduction reactions, crucial for advancements in fields ranging from battery technology to environmental remediation. A new, streamlined approach promises to drastically reduce errors and accelerate scientific discovery.
This method, detailed in a recent publication in "Journal of Chemical Education", offers a step-by-step guide for balancing half-reactions, particularly those occurring in acidic solutions, notorious for their intricate electron and proton transfers.
Balancing Half-Reactions: A Critical Skill
Balancing half-reactions, the building blocks of redox reactions, underpins our understanding of electron transfer processes.
These processes are fundamental to energy storage, corrosion prevention, and the synthesis of countless chemical compounds.
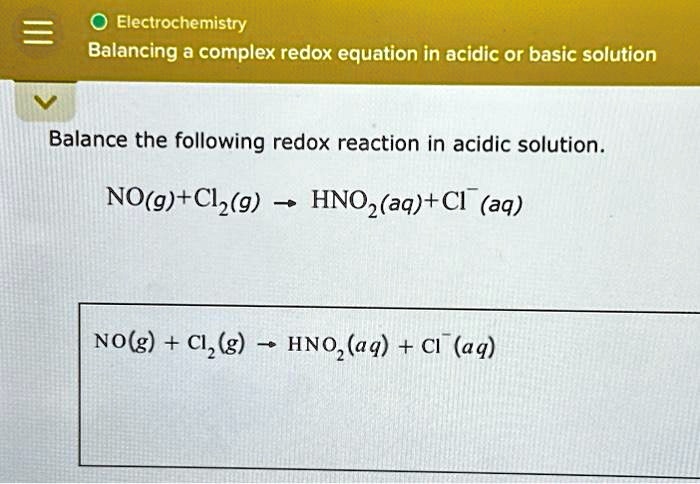
The challenge lies in accurately accounting for all atoms and charges involved, especially in acidic conditions where protons (H+) play a significant role.
The New Method: A Step-by-Step Approach
The core of the new method is a systematic, modular approach, breaking down the balancing process into manageable steps.
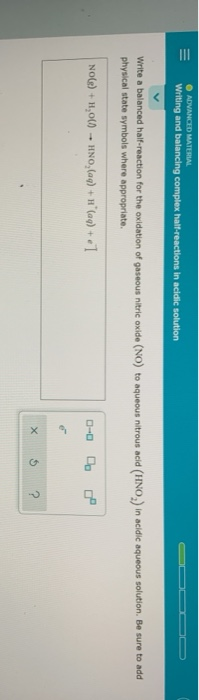
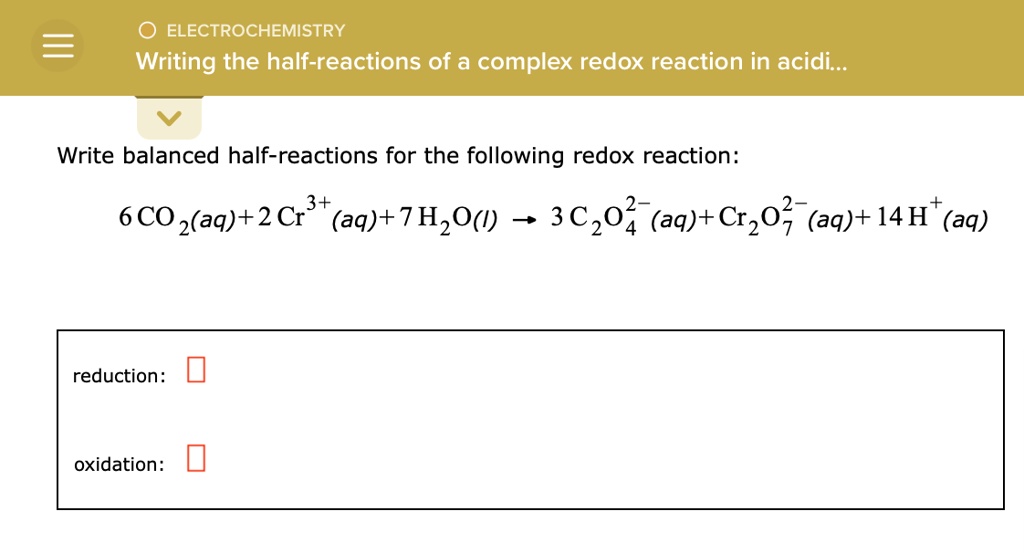
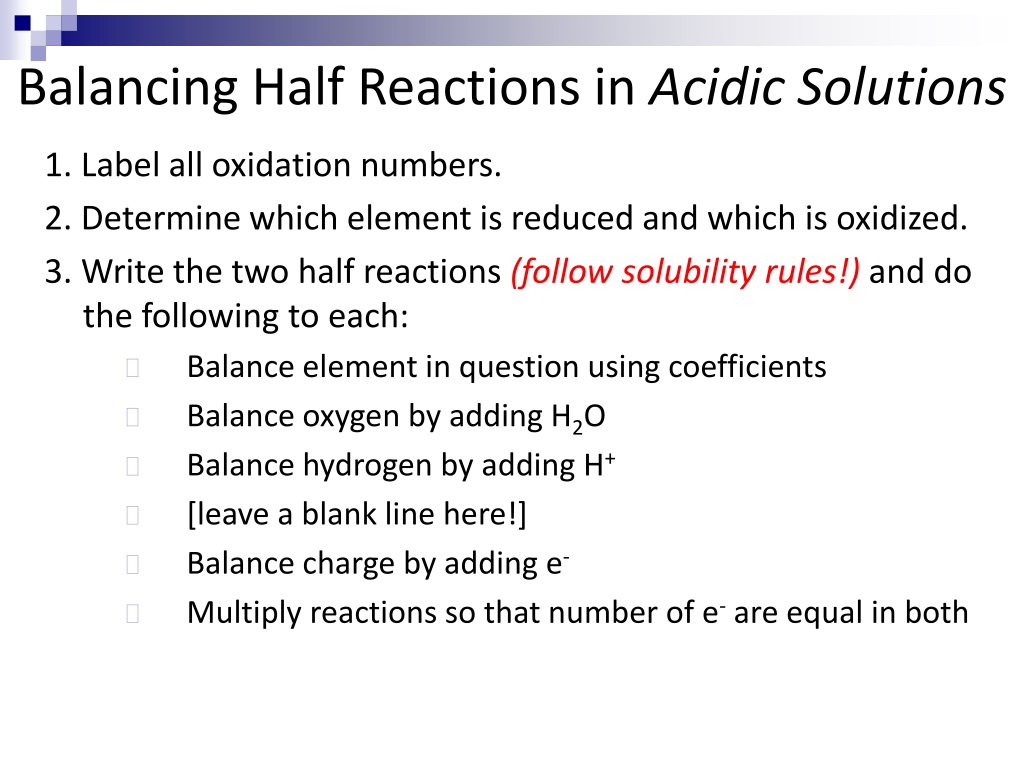
First, identify the elements undergoing oxidation and reduction in the half-reaction.
Then, balance all elements except oxygen and hydrogen.
Next, balance oxygen atoms by adding H2O molecules to the side of the equation that needs more oxygen.
After balancing oxygen, balance hydrogen atoms by adding H+ ions to the side of the equation lacking hydrogen.
Finally, balance the charge by adding electrons (e-) to the side with the more positive charge.
Addressing Common Pitfalls
The traditional method often leads to confusion with the addition of H+ and H2O, particularly when dealing with polyatomic ions.
This new approach emphasizes a clear, logical progression, minimizing the likelihood of errors.
It incorporates visual aids and checklists to ensure each step is completed correctly.
Impact on Education and Research
Dr. Emily Carter, lead author of the study and a professor at the University of California, Berkeley, believes this method will significantly improve chemistry education.
"Students often struggle with balancing redox reactions, which hinders their understanding of electrochemistry and other related fields," Dr. Carter explains.
“Our goal was to develop a method that is both accurate and accessible, empowering students to master this essential skill."
The simplified approach has already shown promising results in preliminary trials with undergraduate chemistry students.
Error rates decreased by an average of 30% compared to students using traditional balancing methods.
Furthermore, students reported a higher level of confidence in their ability to balance complex half-reactions.
Real-World Applications
Beyond education, this method has significant implications for research and industry.
Accurate balancing of redox reactions is crucial in developing new battery technologies, improving the efficiency of fuel cells, and understanding corrosion mechanisms.
It is also essential in environmental remediation, where redox reactions are used to remove pollutants from water and soil.
Examples of Applications
- Battery Development: Optimizing electrode materials and electrolyte compositions relies on precise understanding of redox reactions.
- Corrosion Prevention: Understanding the redox reactions involved in corrosion allows for the development of effective protective coatings.
- Water Treatment: Redox processes are used to remove contaminants like arsenic and chromium from drinking water.
Expert Commentary
"This is a welcome development," says Dr. David Lee, a leading electrochemist at Stanford University, who was not involved in the study.
"Balancing redox reactions can be a tedious and error-prone process, even for experienced researchers."
"A simplified, systematic approach will undoubtedly accelerate progress in various fields that rely on redox chemistry."
Next Steps
Dr. Carter's team is currently developing an online tool that will automatically balance redox reactions using this new method.
This tool will be freely available to students and researchers worldwide.
The team also plans to extend the method to balancing redox reactions in basic solutions, which present their own unique challenges.
The full details of the balancing methodology are available in the current issue of the Journal of Chemical Education, under the title "A Streamlined Approach to Balancing Half-Reactions in Acidic Solution". Further updates and related resources will be posted on the University of California, Berkeley's chemistry department website.






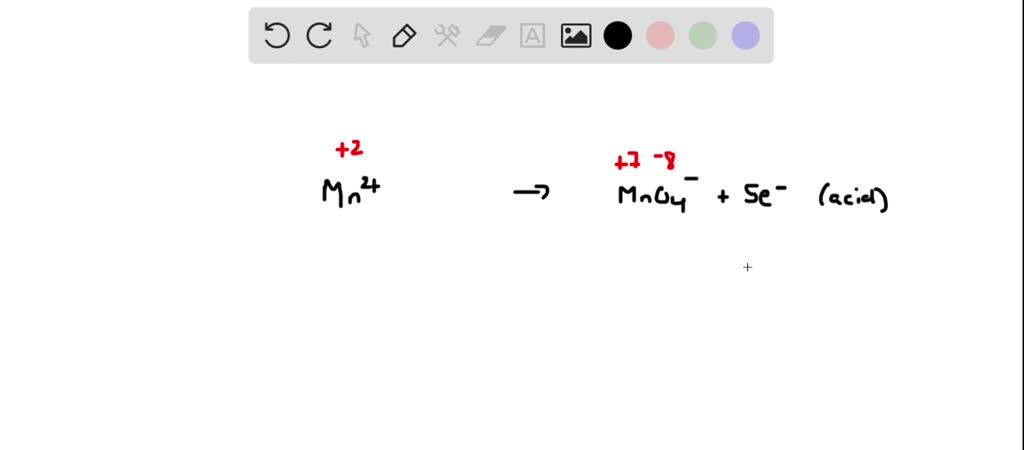

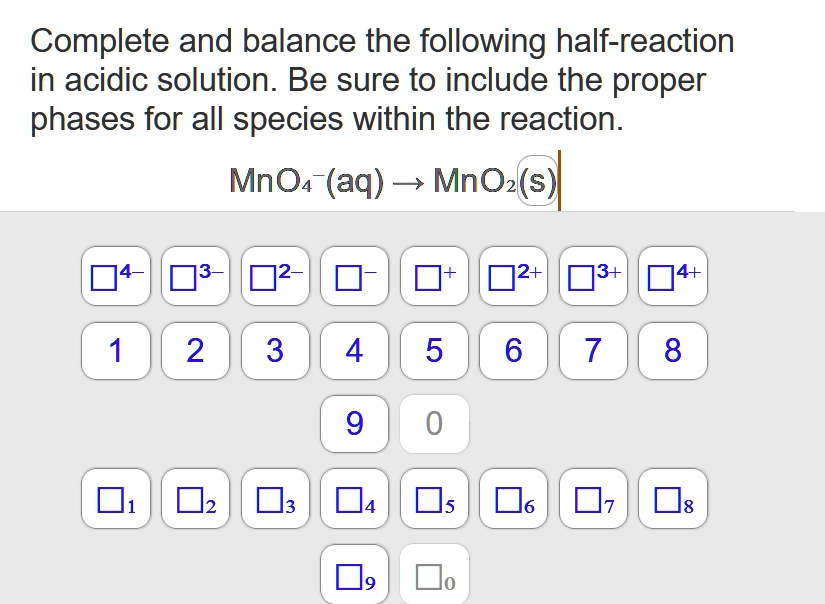
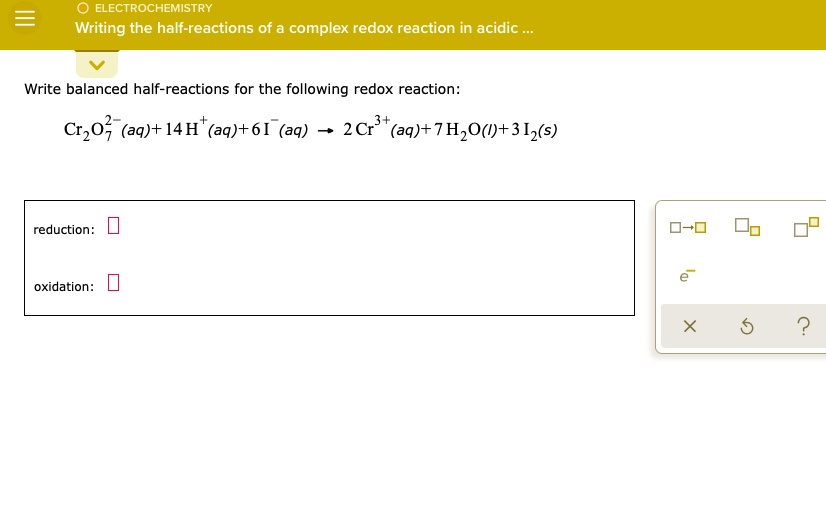
+%2B+NO3-(aq)+%EF%83%A0+Cu2%2B(aq)+%2B+NO2(g)+Acidic+condition..jpg)