3-level Cervical Disc Replacement Fda Approved

Imagine a life where chronic neck pain doesn't dictate your every move. Picture waking up without the familiar, throbbing ache radiating down your arm, numbing your fingers. For countless individuals suffering from debilitating cervical disc disease affecting multiple levels of their spine, this vision of relief is moving closer to reality.
The FDA has recently approved a revolutionary new 3-level cervical disc replacement device, offering a potential game-changer in the treatment of this widespread condition. This approval marks a significant advancement, promising improved quality of life for those previously limited by pain and restricted movement.
Understanding Cervical Disc Disease
Cervical disc disease, a common cause of neck pain and neurological symptoms, arises from the gradual wear and tear of the intervertebral discs in the neck. These discs, which act as cushions between the vertebrae, can degenerate over time due to factors like aging, injury, or genetics. This degeneration can lead to disc herniation, spinal stenosis, and nerve compression, resulting in pain, numbness, weakness, and limited range of motion.
Traditional treatments for cervical disc disease have ranged from conservative measures like physical therapy and pain medication to more invasive procedures such as anterior cervical discectomy and fusion (ACDF). While ACDF can effectively relieve pain, it involves fusing the affected vertebrae, which can limit neck mobility and potentially accelerate degeneration in adjacent discs.
Cervical disc replacement (CDR) emerged as an alternative to ACDF, aiming to preserve neck motion by replacing the damaged disc with an artificial one. Earlier CDR devices focused on single-level or two-level replacements. However, the newly approved 3-level CDR device represents a major leap forward in addressing more complex cases.
The Promise of 3-Level Cervical Disc Replacement
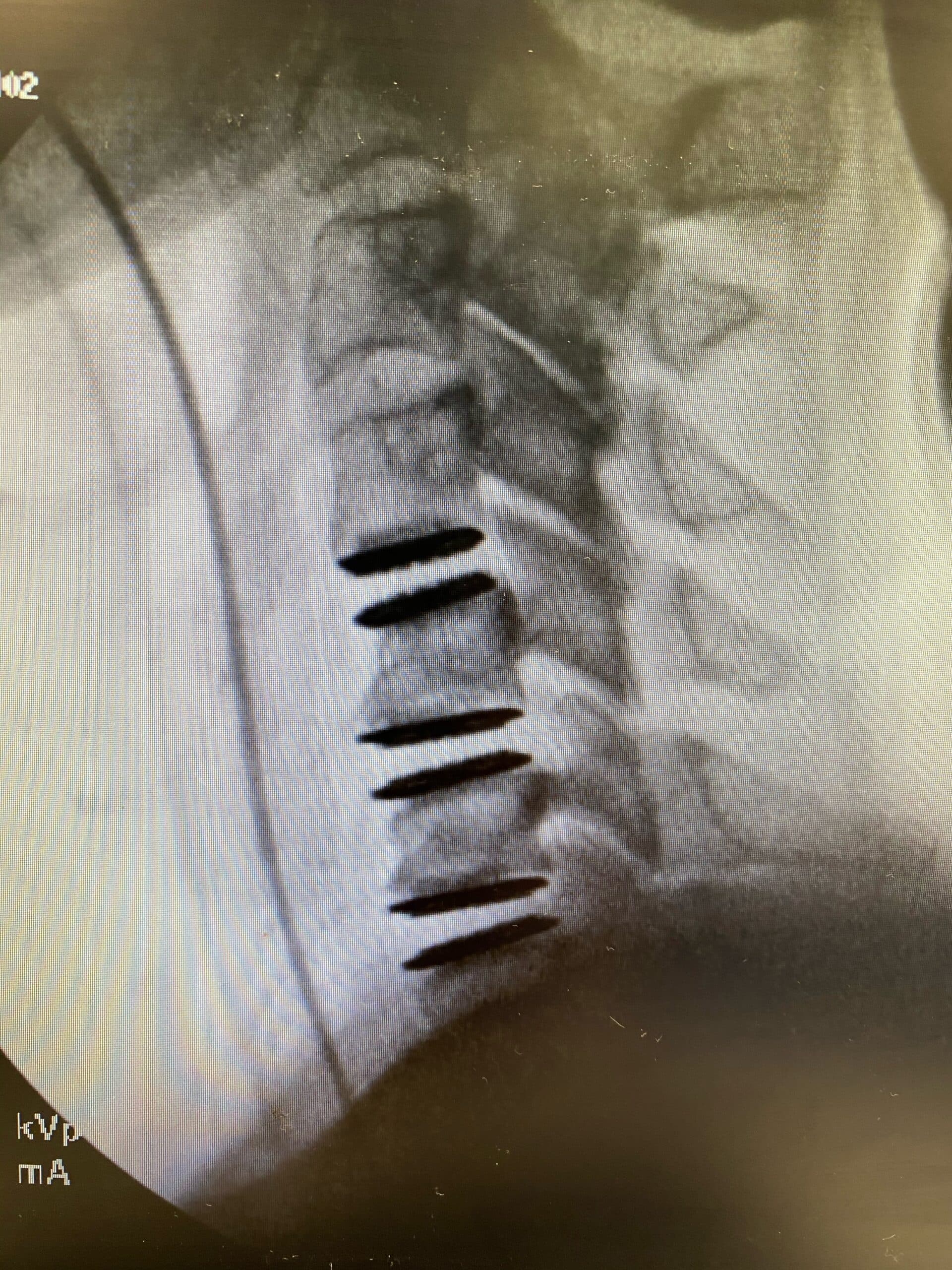
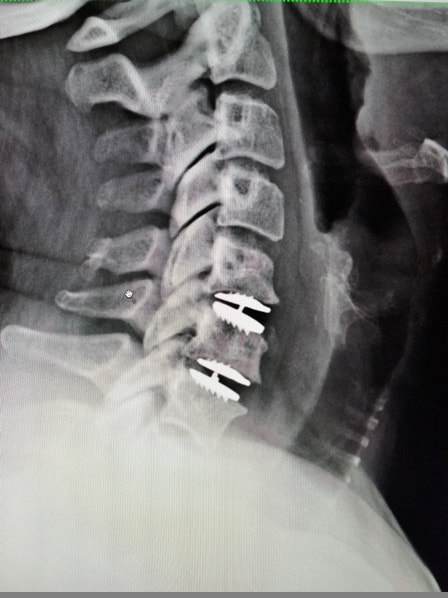
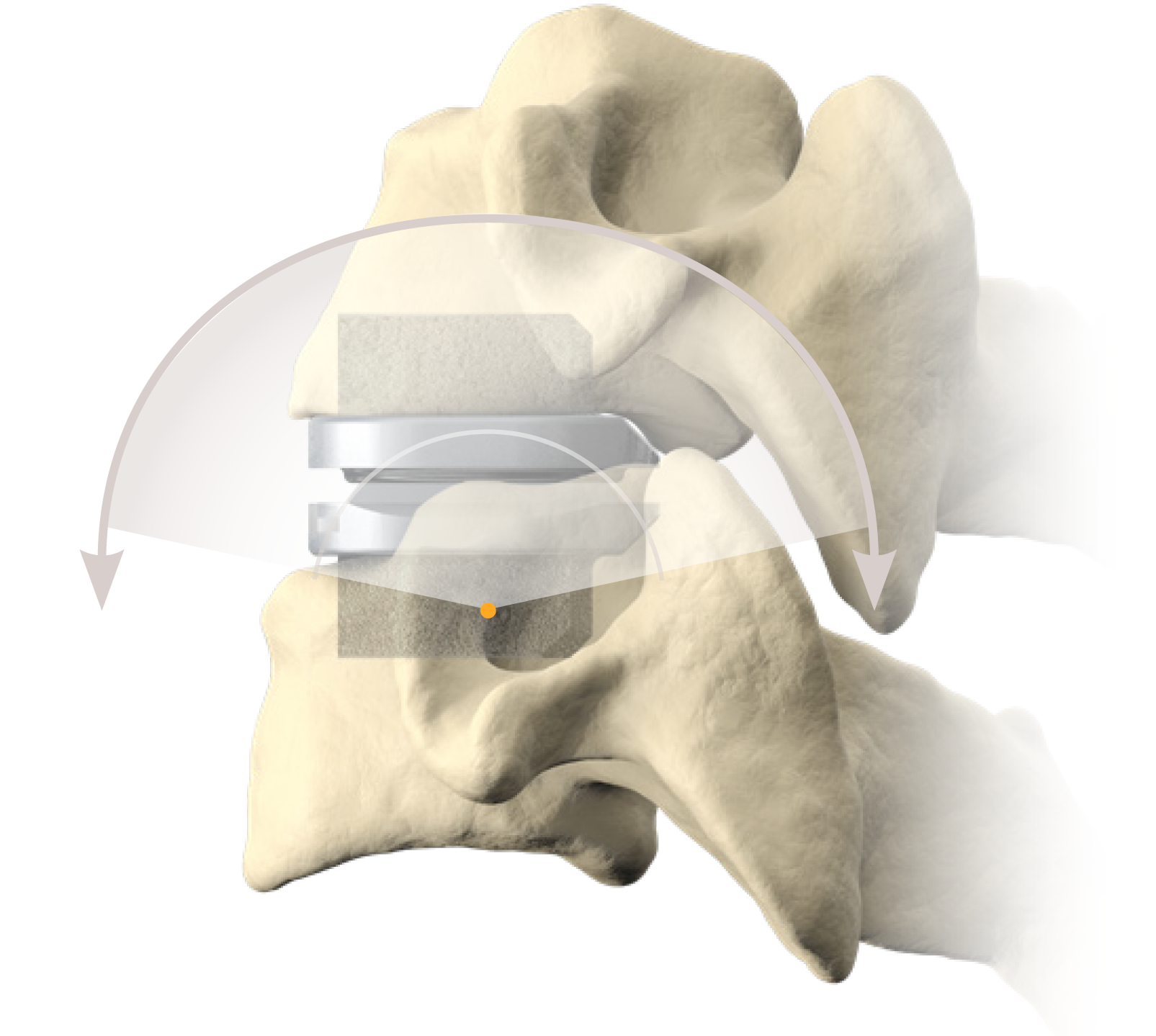
The newly approved device is designed to replace three adjacent cervical discs, maintaining motion at each level. This motion preservation offers a significant advantage over fusion, potentially reducing the risk of adjacent segment degeneration, a common complication following ACDF. By maintaining natural neck movement, the device aims to improve long-term outcomes and overall quality of life for patients.
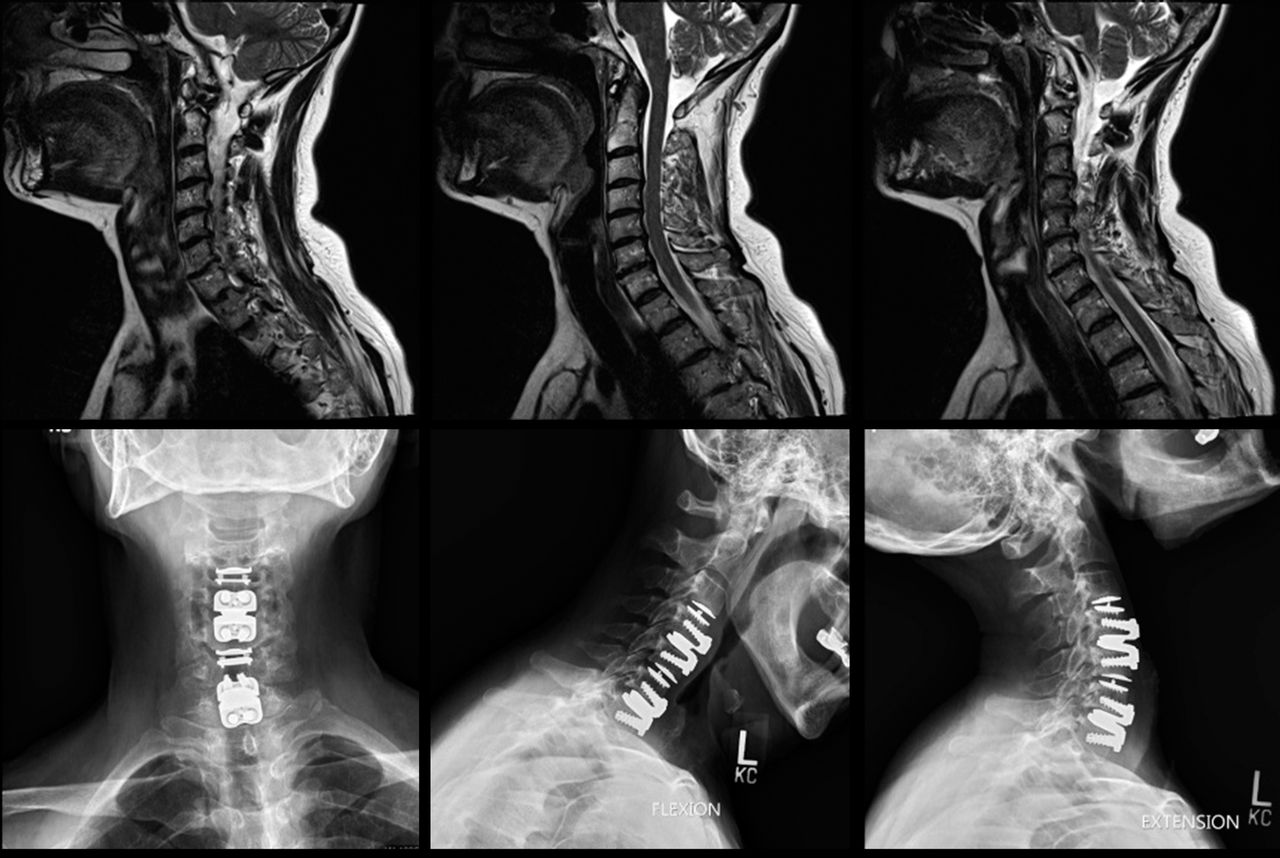
Clinical trials evaluating the safety and efficacy of the 3-level CDR device have demonstrated promising results. Data presented to the FDA showed significant improvements in pain scores, functional status, and overall patient satisfaction compared to ACDF. These findings highlight the potential of the device to provide effective and lasting relief for individuals with multi-level cervical disc disease.
The approval follows rigorous evaluation by the FDA, ensuring the device meets stringent safety and performance standards. This process involves a thorough review of clinical trial data, manufacturing processes, and device design. Ultimately, the FDA's approval signifies confidence in the device's ability to provide a safe and effective treatment option for patients in need.
Implications for Patients and Healthcare Providers
The availability of a 3-level CDR device offers a new treatment option for patients with complex cervical disc disease, potentially improving their quality of life and reducing the need for more invasive procedures. Patients experiencing chronic neck pain, numbness, or weakness affecting multiple levels of their cervical spine may now be eligible for this innovative treatment.
For healthcare providers, the 3-level CDR device expands the treatment options available for managing cervical disc disease. Surgeons can now consider motion-preserving surgery for patients who were previously limited to fusion procedures. This expanded treatment landscape allows for more personalized and patient-centered care.
However, it's crucial to remember that not all patients are suitable candidates for 3-level CDR. Careful patient selection is essential to ensure optimal outcomes. Factors such as bone quality, spinal alignment, and the presence of other underlying conditions must be considered when determining candidacy for the procedure.
A Closer Look at the Device and the Procedure
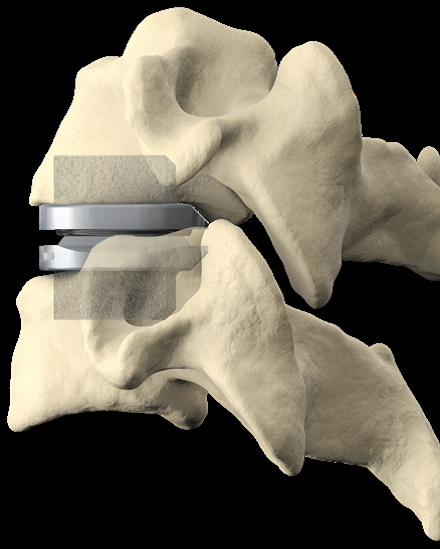
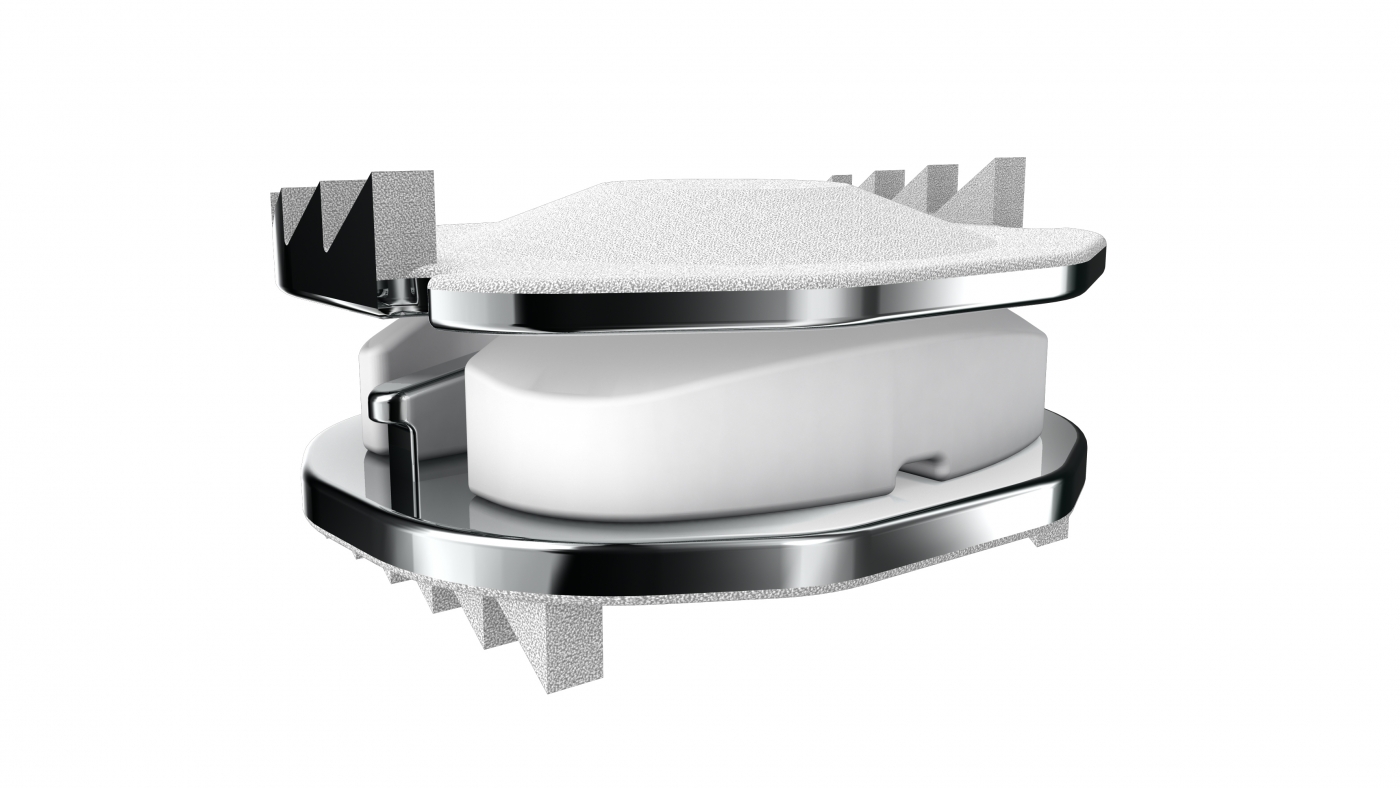
The 3-level CDR device is typically constructed from biocompatible materials like titanium or cobalt-chromium alloy. These materials are chosen for their strength, durability, and compatibility with the human body. The device is designed to mimic the natural movement of the cervical spine, allowing for flexion, extension, rotation, and lateral bending.
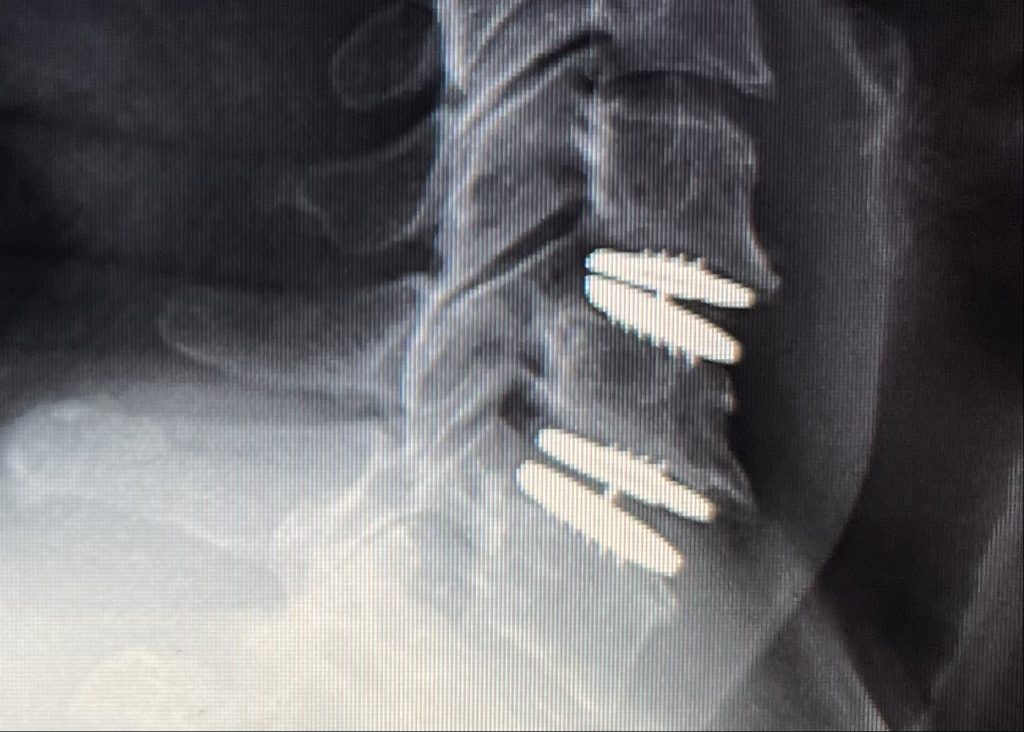
The surgical procedure for implanting the device involves accessing the cervical spine through an anterior (front) approach. The damaged discs are carefully removed, and the artificial discs are inserted into the spaces between the vertebrae. The device is then secured in place using specialized instruments.
Post-operative rehabilitation is crucial for ensuring optimal outcomes following 3-level CDR. Patients typically undergo physical therapy to regain strength, flexibility, and range of motion in their neck. Adherence to post-operative instructions is essential for proper healing and device integration.
Looking Ahead: The Future of Cervical Spine Care
The approval of the 3-level CDR device represents a significant step forward in the evolution of cervical spine care. This innovation underscores the ongoing efforts to develop less invasive, motion-preserving treatments for spinal disorders. As technology continues to advance, we can expect to see further refinements in CDR devices and surgical techniques.
Future research will likely focus on long-term outcomes following 3-level CDR, comparing its effectiveness to ACDF over extended periods. Studies will also explore the potential for combining CDR with other therapies, such as biologics, to enhance spinal healing and regeneration.
Ultimately, the goal is to provide patients with the most effective and least invasive treatment options for managing cervical disc disease, allowing them to return to active and fulfilling lives. The FDA approval of the 3-level CDR device marks a significant milestone in this journey, bringing hope and improved quality of life to countless individuals suffering from chronic neck pain.
The journey toward a pain-free life is a personal one, filled with both challenges and triumphs. As this new technology becomes more widely available, it offers a beacon of hope for those navigating the complexities of cervical disc disease. It's a reminder that advancements in medicine are continually working to improve our well-being, one innovative solution at a time.