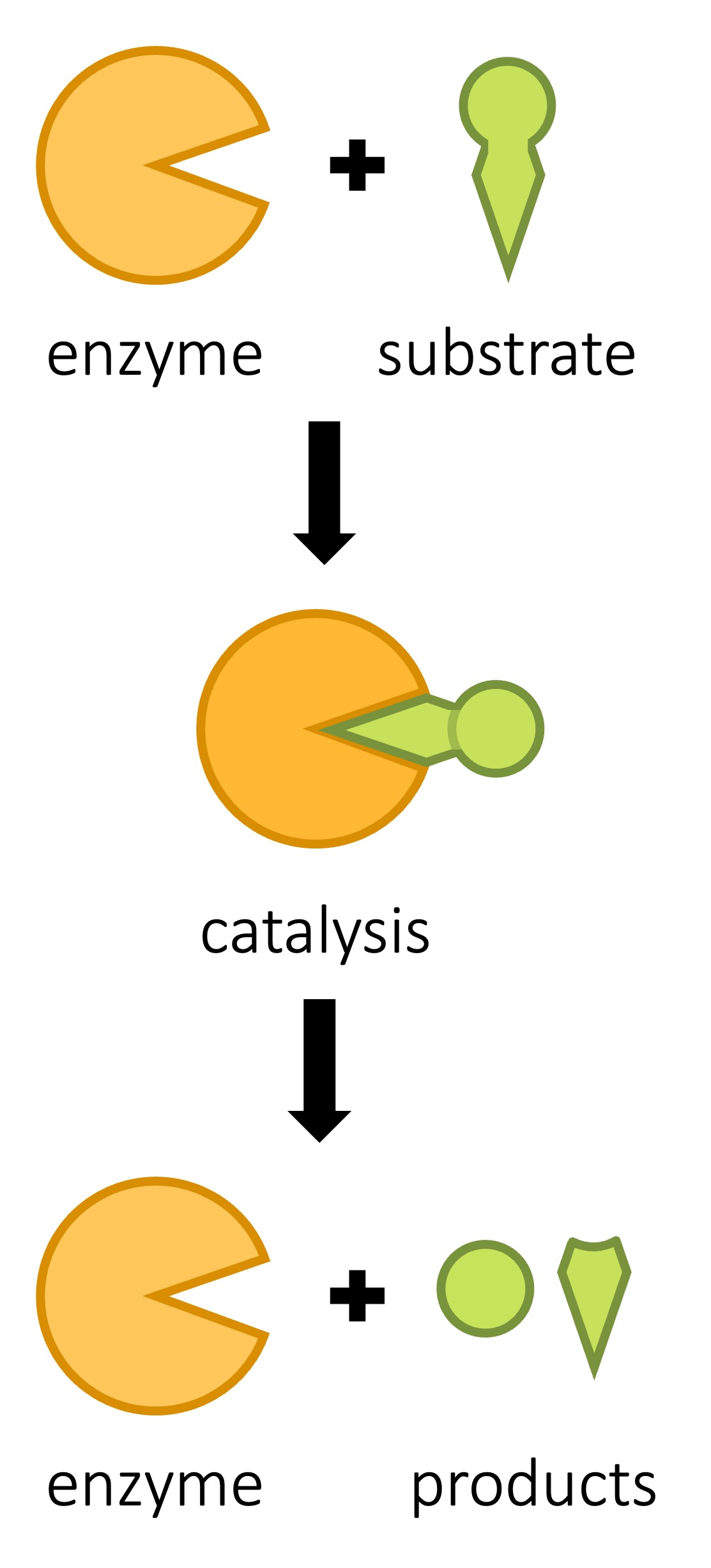
After An Enzyme Reaction Is Completed The Enzyme
The biological world hums with activity, driven by intricate molecular machines called enzymes. These tireless catalysts accelerate life's chemical reactions, but what happens to them after their work is done? The fate of an enzyme after it facilitates a reaction is far from passive; it's a carefully orchestrated process critical for cellular efficiency and regulation.
Understanding the post-reaction life of an enzyme – its release of products, potential for reuse, susceptibility to modification, and eventual degradation – is crucial for comprehending cellular metabolism, drug action, and various biotechnological applications. The enzyme's journey doesn't end with product formation; instead, it enters a new phase governed by cellular signals and environmental cues, impacting overall biochemical pathways.
Enzyme Recycling and Reuse
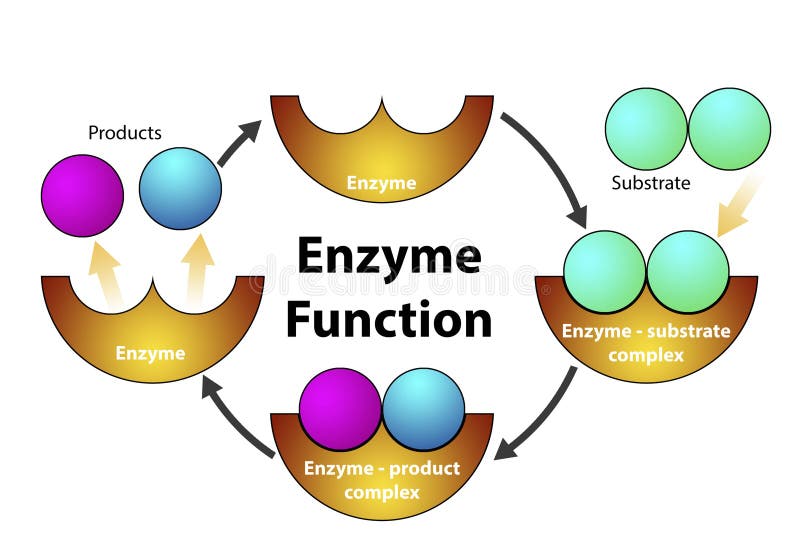
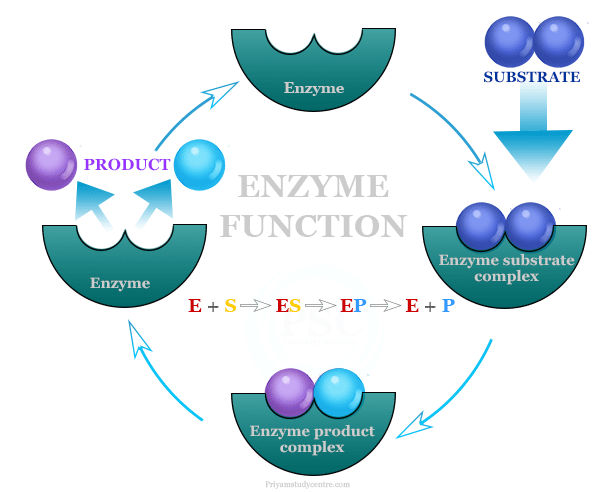
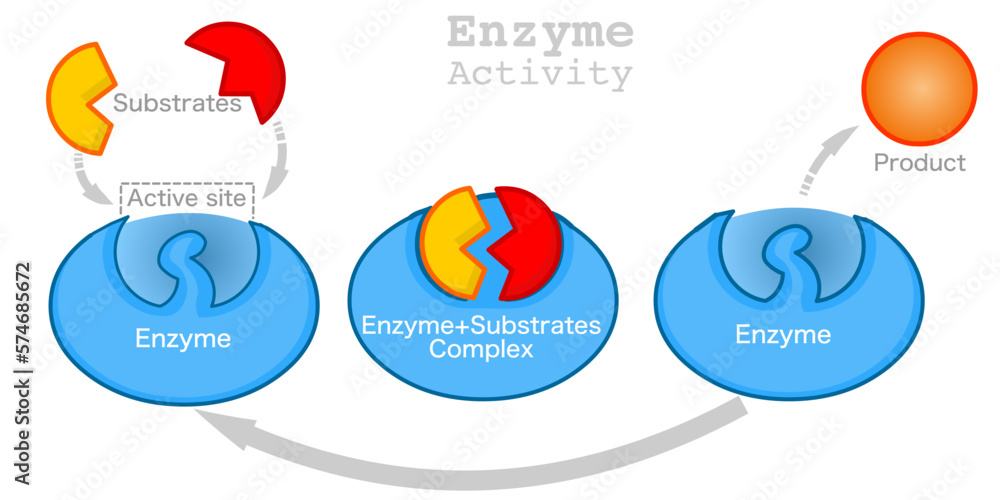
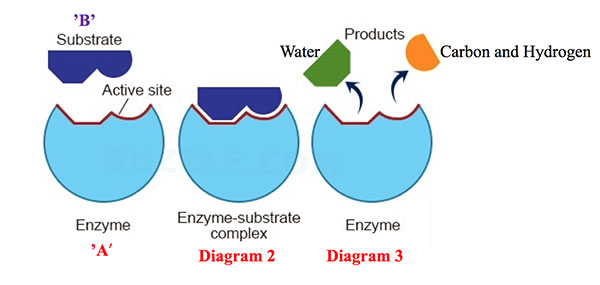
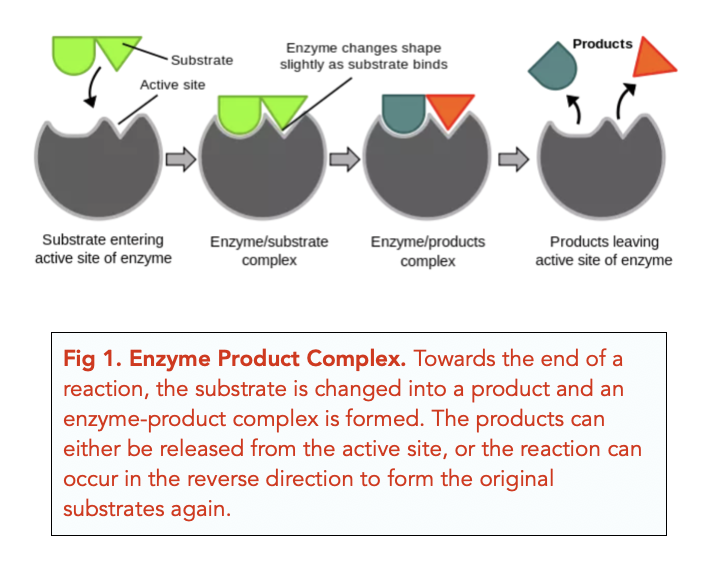
One of the most remarkable aspects of enzymes is their ability to catalyze the same reaction repeatedly. After an enzyme facilitates a chemical reaction and generates the product, it detaches from the product molecule. The enzyme is then ready to bind to another substrate molecule and begin the catalytic cycle anew.
This process of enzyme recycling is incredibly efficient. It allows a single enzyme molecule to catalyze the conversion of numerous substrate molecules, drastically accelerating the rate of biochemical reactions within the cell.
Enzyme Regulation and Modification
The activity of enzymes isn't constant; it's meticulously regulated to meet the cell's changing needs. This regulation can occur through various mechanisms, including allosteric control and covalent modification. Allosteric regulation involves the binding of a molecule (an activator or inhibitor) to a site distinct from the active site, altering the enzyme's shape and activity.
Covalent modification, on the other hand, involves the addition or removal of a chemical group to the enzyme. Phosphorylation, the addition of a phosphate group, is a common example. These modifications can either activate or inhibit the enzyme, providing a rapid and reversible means of control.
Enzyme Degradation
While enzymes are reusable, they aren't immortal. Enzymes, like all proteins, are subject to degradation. This process is essential for removing damaged or misfolded enzymes, as well as for controlling the overall levels of specific enzymes within the cell.
The primary pathway for protein degradation in eukaryotic cells is the ubiquitin-proteasome system. In this system, enzymes are tagged with ubiquitin, a small protein that signals the proteasome, a protein complex, to degrade the tagged enzyme.
Enzyme degradation is also influenced by environmental factors such as temperature, pH, and the presence of proteases. Extreme conditions can denature enzymes, rendering them non-functional and more susceptible to degradation.
The Importance of Enzyme Turnover
The balance between enzyme synthesis and degradation, known as enzyme turnover, is crucial for maintaining cellular homeostasis. This delicate balance ensures that the cell has the right amount of each enzyme at any given time.
Dysregulation of enzyme turnover can lead to various diseases. For example, the accumulation of misfolded proteins, including enzymes, can contribute to neurodegenerative disorders.
Enzymes in Biotechnology and Medicine
The understanding of enzyme behavior after reaction completion has significant implications for biotechnology and medicine. Enzymes are widely used in industrial processes, such as food production and biofuel synthesis.
The ability to engineer enzymes with improved stability, activity, and substrate specificity is a major goal in biotechnology. Researchers are also developing novel strategies for enzyme immobilization, which can enhance their reusability and stability in industrial settings.
In medicine, enzymes are used as diagnostic tools and therapeutic agents. Enzyme inhibitors are a major class of drugs. Understanding how these inhibitors interact with enzymes after the initial reaction is crucial for designing more effective and targeted therapies.
Future Directions
Research continues to unravel the intricate details of enzyme regulation and degradation. Scientists are employing advanced techniques such as proteomics and structural biology to gain a deeper understanding of these processes.
A key area of focus is the development of new technologies for monitoring enzyme activity in real-time within living cells. This would provide valuable insights into the dynamic regulation of metabolic pathways and the impact of various environmental factors.
Ultimately, a comprehensive understanding of the enzyme lifecycle, from synthesis to degradation, will pave the way for new biotechnological innovations and improved therapeutic strategies. It promises a future where we can harness the power of these biological catalysts with unprecedented precision and efficiency.


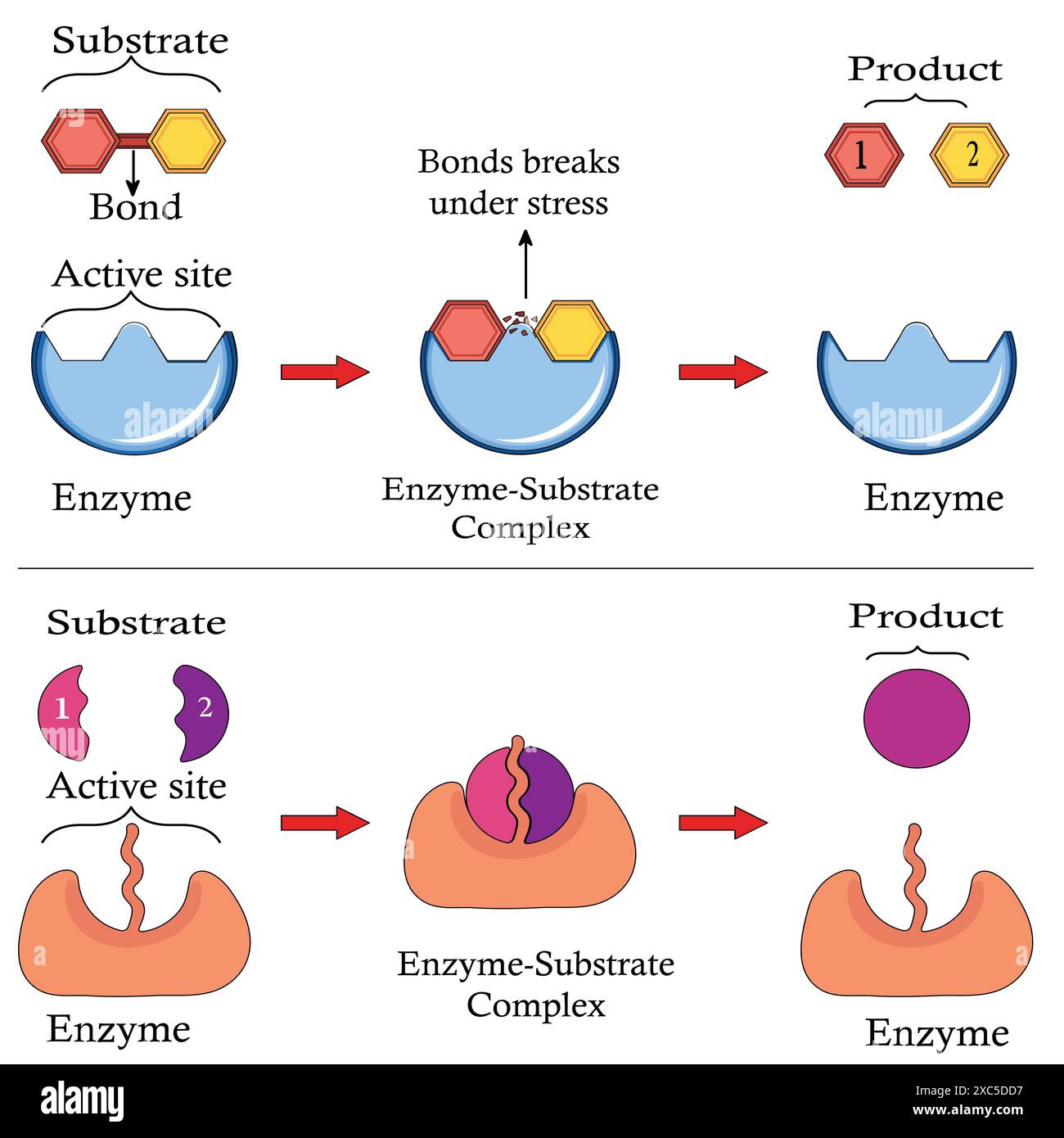


..jpg)