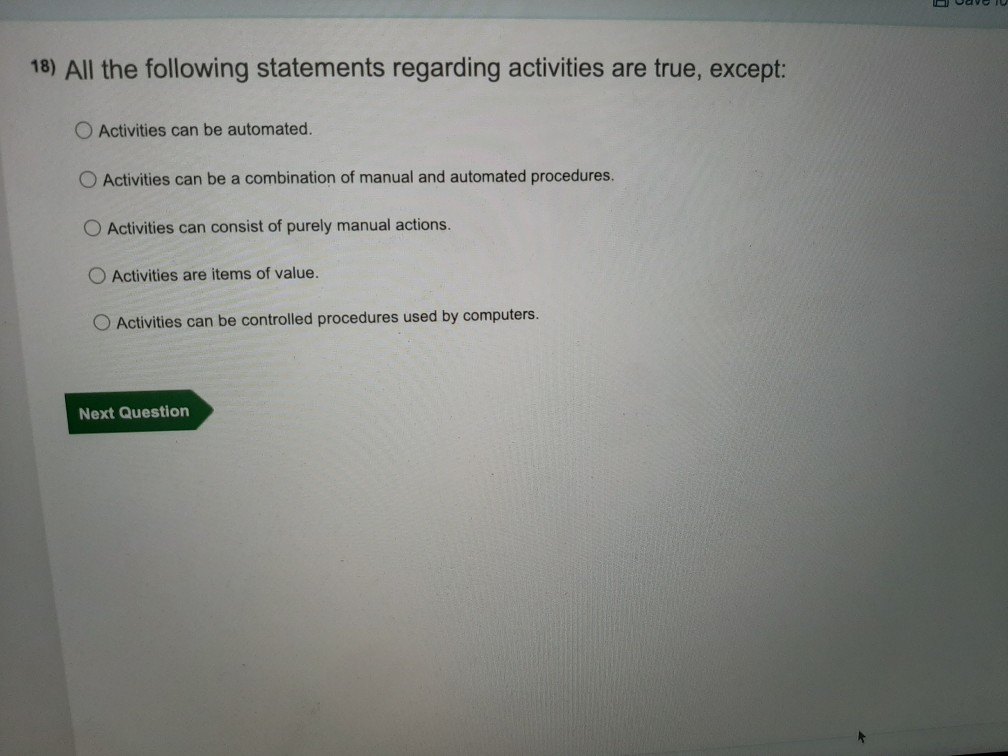
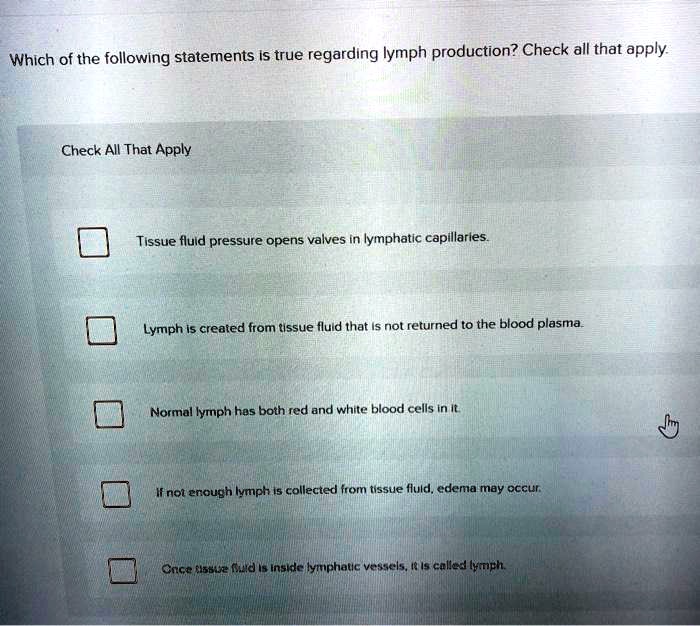
All Of The Following Statements Regarding Ph Are True Except:

The concept of pH, a seemingly simple measure of acidity and alkalinity, underpins a vast range of scientific disciplines, from environmental science and medicine to agriculture and food processing. However, misconceptions about pH abound, often leading to flawed interpretations and potentially harmful applications.
This article aims to dissect common misunderstandings surrounding pH, clarifying its true nature while dispelling widespread myths. We'll explore a statement that might appear correct at first glance but contains a critical flaw, leading to a deeper understanding of this fundamental chemical property.
Understanding pH: Beyond the Basics
pH, short for "potential of hydrogen," is a logarithmic scale used to specify the acidity or basicity of an aqueous solution. It ranges from 0 to 14, with 7 indicating neutrality. Values below 7 are acidic, and values above 7 are alkaline or basic.
The pH scale is logarithmic, meaning that each whole pH value below 7 is ten times more acidic than the next higher value. Similarly, each whole pH value above 7 is ten times more alkaline than the next lower value.
This logarithmic relationship is crucial for understanding the impact of even small changes in pH on chemical and biological systems.
Debunking the Myth: A Critical Look
Let's address the core of the issue: "All of the following statements regarding pH are true except: A neutral solution has a pH of 7 at all temperatures." This statement, while seemingly accurate, contains a critical nuance that renders it false.
While a pH of 7 signifies neutrality under standard conditions (25°C or 77°F), this is not universally true. Temperature significantly affects the dissociation of water, altering the concentration of hydrogen (H+) and hydroxide (OH-) ions.
Neutrality is defined as the point where the concentration of H+ ions equals the concentration of OH- ions. However, the actual numerical pH value at which this occurs changes with temperature.
The Impact of Temperature on pH
As temperature increases, the dissociation of water also increases. This means that even in pure water, the concentrations of both H+ and OH- ions rise.
However, the concentrations remain equal, maintaining neutrality. The pH, however, shifts to a lower value.
For example, at higher temperatures, a solution with a pH slightly below 7 may actually be neutral. This is because the concentration of H+ and OH- ions are equal at that specific temperature.
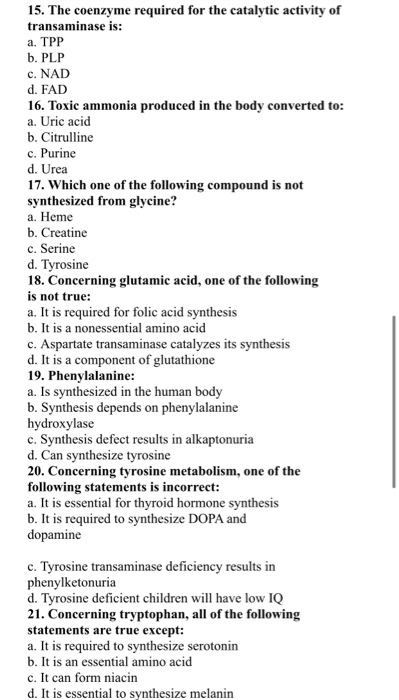
Why This Matters: Practical Implications
Understanding the temperature dependence of pH is crucial in various scientific and industrial contexts. In environmental monitoring, accurate pH measurements of water samples are essential for assessing water quality.
Temperature fluctuations in natural bodies of water can significantly affect pH readings, impacting aquatic life and chemical processes. Ignoring temperature effects can lead to inaccurate assessments and flawed conclusions.
Similarly, in chemical reactions and industrial processes, controlling temperature is vital for maintaining the desired pH and ensuring optimal outcomes. Many reactions are highly pH-dependent.
Biological Systems and pH
Living organisms are highly sensitive to pH changes, and maintaining stable pH levels within cells and tissues is critical for survival. The human body, for example, tightly regulates blood pH within a narrow range (7.35-7.45).
Enzymes, the biological catalysts that drive biochemical reactions, are particularly sensitive to pH. Each enzyme has an optimal pH range at which it functions most effectively. Deviations from this optimal range can impair enzyme activity.
Temperature also plays a role in enzyme activity and pH regulation in biological systems. Understanding these interactions is crucial for studying biological processes and developing medical treatments.
The Importance of Precise Measurement
Accurate pH measurement is essential across a wide range of applications. pH meters are commonly used to determine pH, but proper calibration and maintenance are crucial for reliable results.
Temperature compensation is a critical feature in many pH meters, allowing for accurate measurements at varying temperatures. It is important to be aware of the limitations of pH meters and to use appropriate techniques for different types of samples.
Furthermore, the choice of buffer solutions used for calibration can also affect the accuracy of pH measurements. Selecting appropriate buffers and following recommended calibration procedures are essential for obtaining reliable results.
Looking Ahead: Continuous Learning
The seemingly simple concept of pH reveals surprising complexity upon closer examination. Understanding the temperature dependence of pH is essential for anyone working in fields that rely on accurate pH measurements.
Continuous learning and critical evaluation of scientific information are essential for avoiding misconceptions and ensuring the responsible application of scientific knowledge. Misunderstandings about basic scientific principles can have significant consequences.
By debunking common myths and promoting a deeper understanding of pH, we can contribute to more informed decision-making and improved outcomes in a variety of fields.








![All Of The Following Statements Regarding Ph Are True Except: [ANSWERED] All of the following statements are correct regarding - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20211203063233722844-3475341.jpg?h=512)