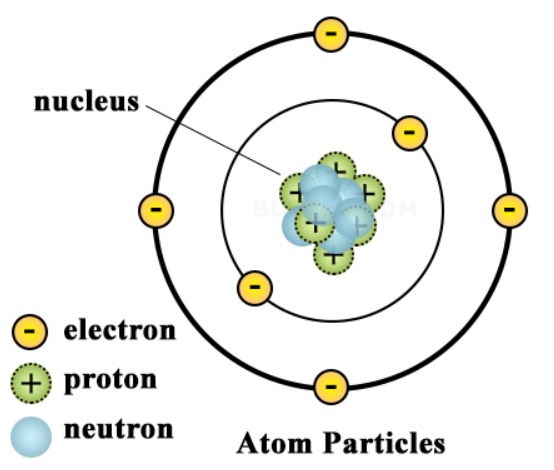
An Atom Is Best Described As

For decades, the image of an atom has been cemented in textbooks: a central nucleus orbited by electrons, much like planets around a sun. However, the reality is far more nuanced and probabilistic, prompting physicists and educators to refine our understanding of the fundamental building block of matter.
This article explores the evolving definition of an atom, delving into the quantum mechanical model and its implications for how we perceive and interact with the world around us. The shift in understanding isn't just academic; it impacts fields ranging from materials science to quantum computing.
Challenging the Planetary Model
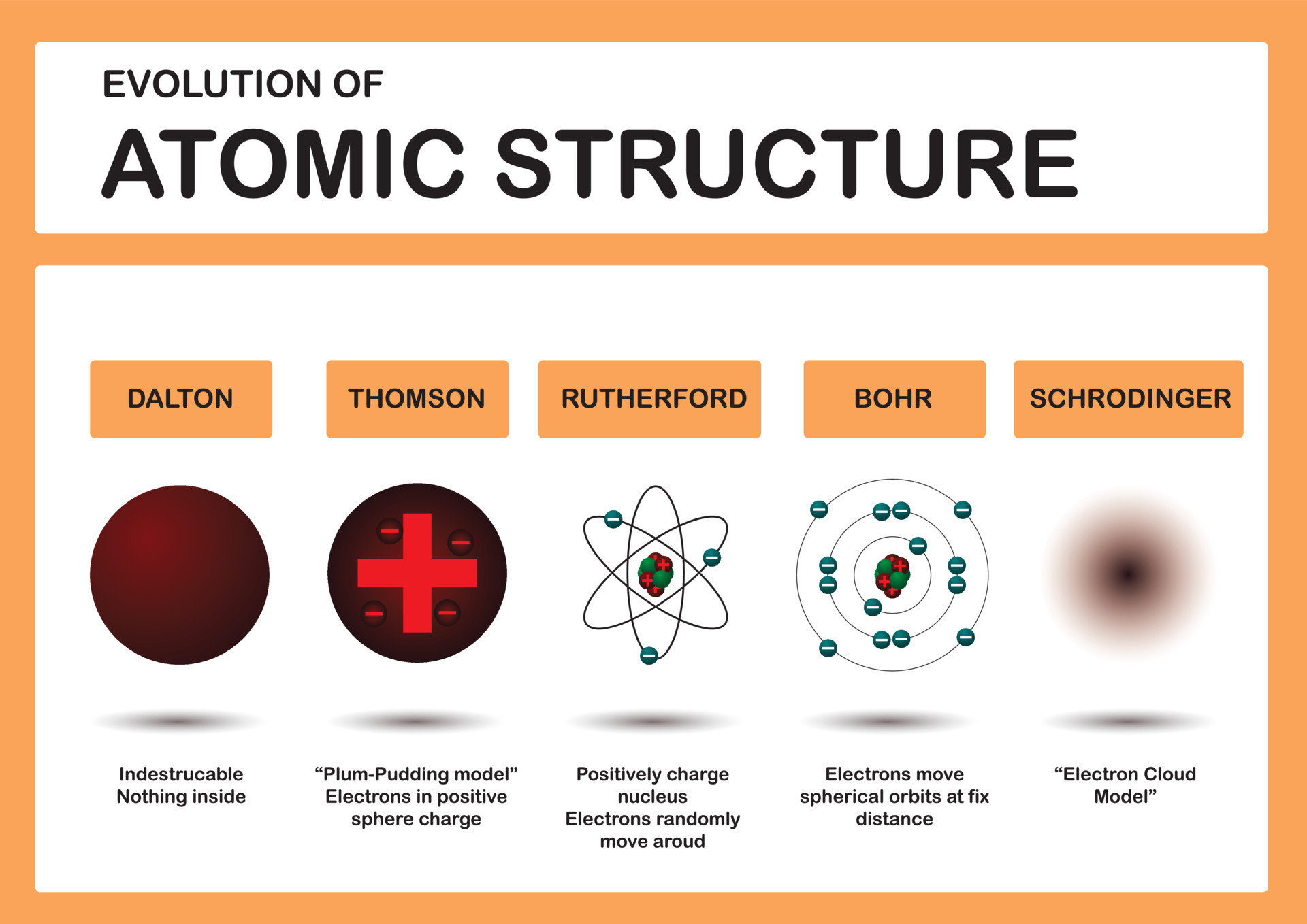
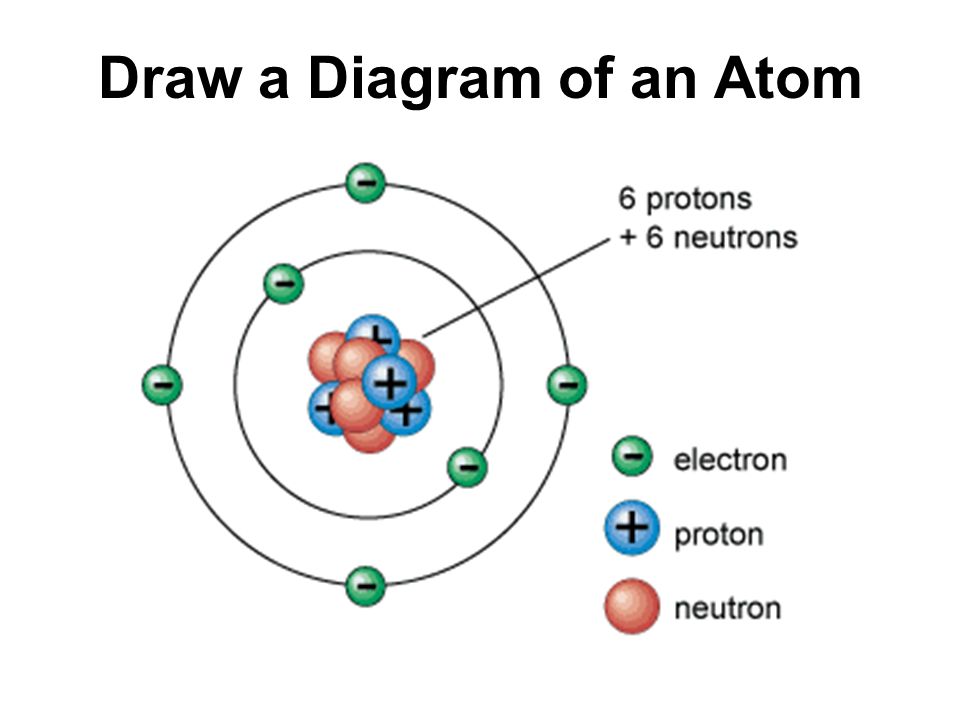
The traditional Bohr model, introduced in the early 20th century, provided a simple, intuitive visualization of atomic structure. It depicted electrons traveling in fixed orbits around the nucleus, akin to planets around the Sun. While helpful for introductory explanations, this model fails to capture the true behavior of electrons at the atomic level.
The advent of quantum mechanics revolutionized our understanding. Quantum mechanics reveals that electrons do not orbit the nucleus in neat, defined paths.
Instead, they exist in probability clouds, or atomic orbitals, representing the likelihood of finding an electron in a particular region of space. This concept challenges the classical idea of a fixed trajectory.
The Quantum Mechanical Atom
The modern description of an atom is rooted in the solutions to the Schrödinger equation, a fundamental equation in quantum mechanics. These solutions define the atomic orbitals, which are characterized by specific energy levels and shapes.
These orbitals represent the probability distribution of an electron’s location, rather than a defined path. The shapes of these orbitals are often complex, ranging from spherical (s orbitals) to dumbbell-shaped (p orbitals) and more intricate forms (d and f orbitals).
Understanding these orbitals is crucial for predicting and explaining chemical bonding. The way these orbitals interact determines how atoms combine to form molecules and materials.
Beyond the Basics: The Nucleus
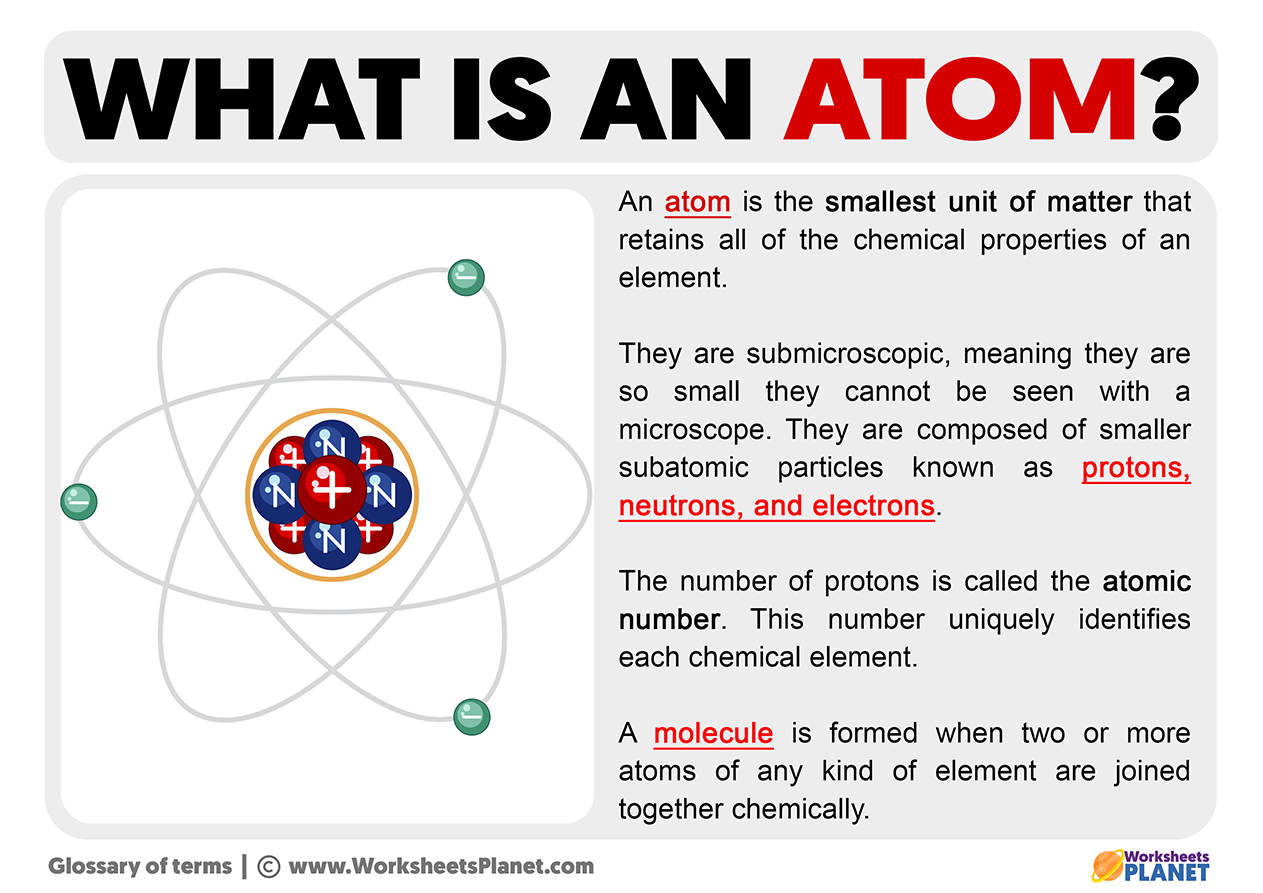
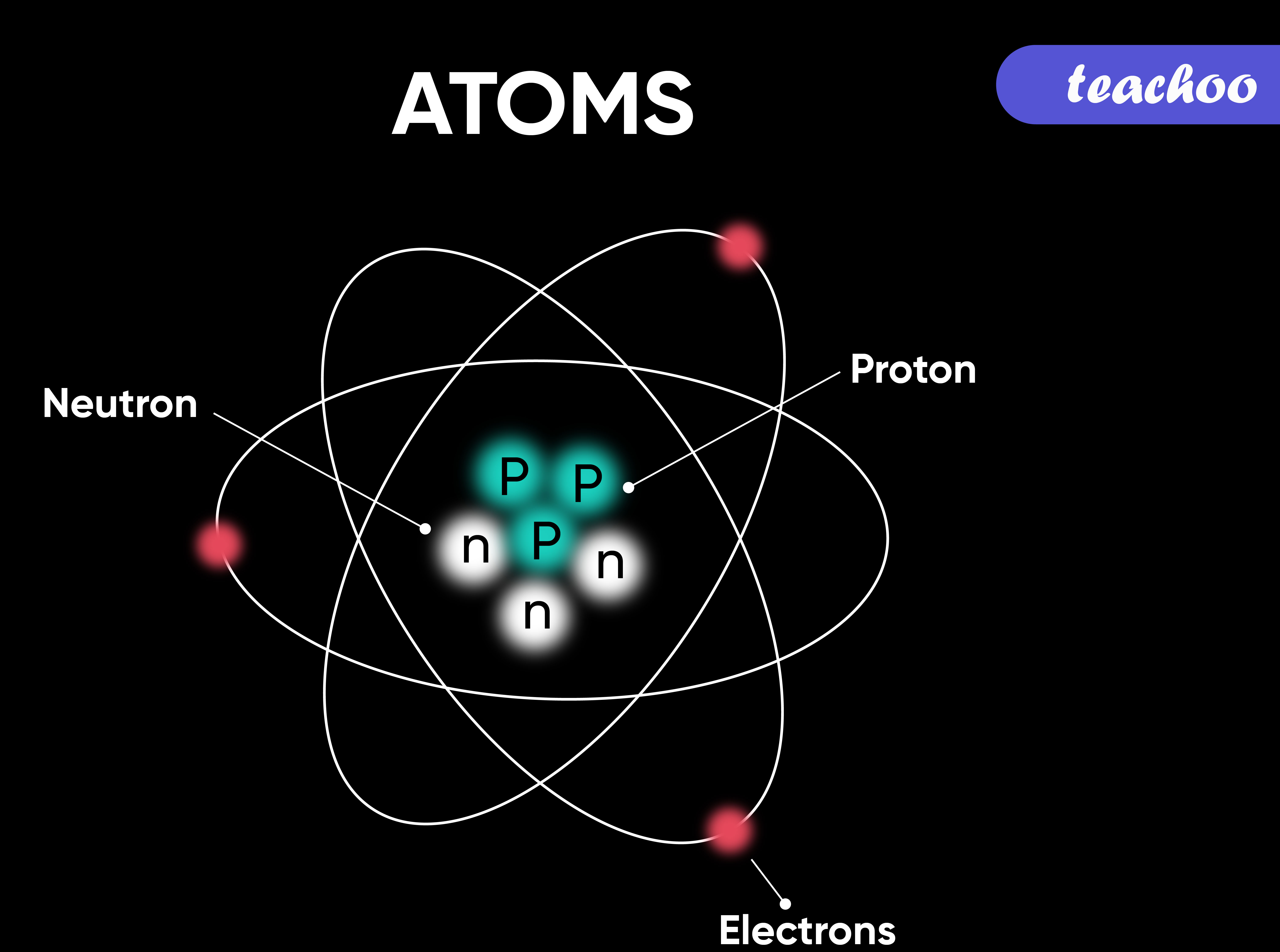
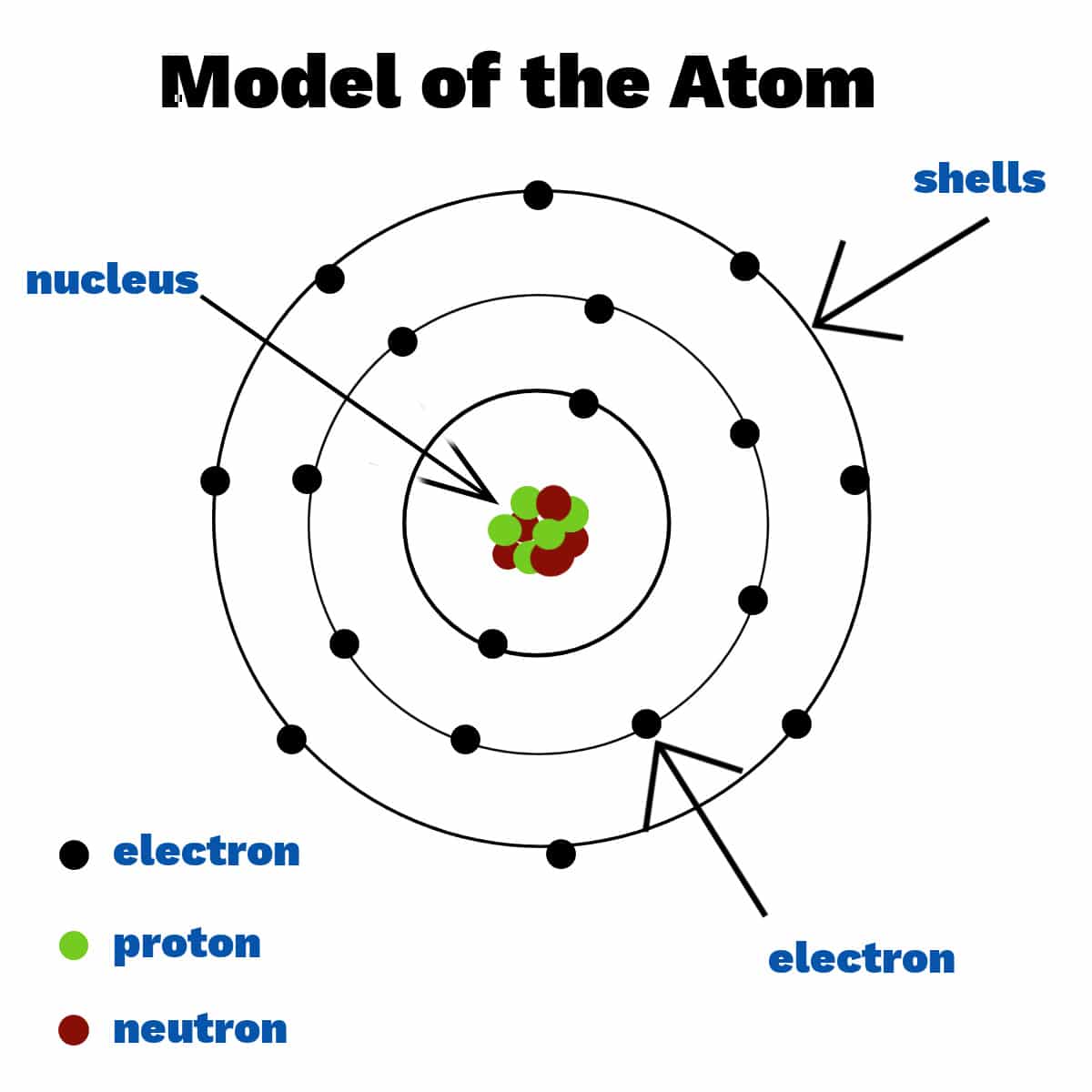
The nucleus, located at the center of the atom, contains protons and neutrons. Protons carry a positive charge, while neutrons are electrically neutral. The number of protons defines the element to which the atom belongs (e.g., all atoms with one proton are hydrogen atoms).
The strong nuclear force holds the protons and neutrons together within the nucleus. This force is incredibly powerful, overcoming the electrostatic repulsion between the positively charged protons.
Isotopes are atoms of the same element that have different numbers of neutrons. While they have the same chemical properties, their differing masses can affect their physical properties and stability.
The Significance of Electron Configuration
The arrangement of electrons within an atom’s orbitals, known as its electron configuration, dictates its chemical behavior. Atoms tend to gain, lose, or share electrons to achieve a stable electron configuration, typically resembling that of a noble gas.
This drive towards stability is the foundation of chemical bonding. Atoms interact with each other to form molecules and compounds, driven by the desire to minimize their energy.
Understanding electron configuration allows chemists to predict the types of bonds an atom will form and the properties of the resulting compounds. This is crucial in designing new materials and pharmaceuticals.
Impact and Applications
The quantum mechanical model of the atom has had a profound impact on various fields. In materials science, it allows for the design of materials with specific properties, such as high strength or conductivity.
In chemistry, it provides a framework for understanding chemical reactions and designing new catalysts. In quantum computing, the quantum properties of atoms are harnessed to create powerful computational devices.
Furthermore, medical imaging techniques like MRI rely on the quantum mechanical properties of atomic nuclei to generate detailed images of the human body.
The Future of Atomic Understanding
Research into the atom is ongoing, with scientists continually refining our understanding of its behavior. For example, the study of exotic atoms, where electrons are replaced by other particles, provides insights into the fundamental forces of nature.
Advances in microscopy allow scientists to image atoms with unprecedented resolution. These images confirm the predictions of quantum mechanics and reveal the intricate details of atomic structure.
The exploration of quantum entanglement and other quantum phenomena could lead to even more revolutionary technologies in the future.
Conclusion
The atom, once conceived as a miniature solar system, is now understood to be a complex entity governed by the laws of quantum mechanics. This shift in understanding has revolutionized science and technology, leading to countless innovations.
While the classical model may still be useful for introductory purposes, it is crucial to recognize its limitations and embrace the more accurate and nuanced quantum mechanical model.
As research continues, our understanding of the atom will undoubtedly evolve, unlocking even more potential for scientific discovery and technological advancement. The atom, despite its minute size, holds the key to unlocking some of the universe's greatest mysteries.









![An Atom Is Best Described As [DIAGRAM] Labeled Diagram Of Atomic Structure - MYDIAGRAM.ONLINE](https://showme0-9071.kxcdn.com/files/637037/pictures/thumbs/2451040/last_thumb1473726440.jpg)